Coordinated reset stimulation of plastic neural networks with spatially dependent synaptic connections
- PMID: 38863734
- PMCID: PMC11165135
- DOI: 10.3389/fnetp.2024.1351815
Coordinated reset stimulation of plastic neural networks with spatially dependent synaptic connections
Abstract
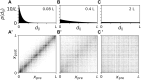
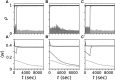
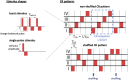
Background: Abnormal neuronal synchrony is associated with several neurological disorders, including Parkinson's disease (PD), essential tremor, dystonia, and epilepsy. Coordinated reset (CR) stimulation was developed computationally to counteract abnormal neuronal synchrony. During CR stimulation, phase-shifted stimuli are delivered to multiple stimulation sites. Computational studies in plastic neural networks reported that CR stimulation drove the networks into an attractor of a stable desynchronized state by down-regulating synaptic connections, which led to long-lasting desynchronization effects that outlasted stimulation. Later, corresponding long-lasting desynchronization and therapeutic effects were found in animal models of PD and PD patients. To date, it is unclear how spatially dependent synaptic connections, as typically observed in the brain, shape CR-induced synaptic downregulation and long-lasting effects.
Methods: We performed numerical simulations of networks of leaky integrate-and-fire neurons with spike-timing-dependent plasticity and spatially dependent synaptic connections to study and further improve acute and long-term responses to CR stimulation.
Results: The characteristic length scale of synaptic connections relative to the distance between stimulation sites plays a key role in CR parameter adjustment. In networks with short synaptic length scales, a substantial synaptic downregulation can be achieved by selecting appropriate stimulus-related parameters, such as the stimulus amplitude and shape, regardless of the employed spatiotemporal pattern of stimulus deliveries. Complex stimulus shapes can induce local connectivity patterns in the vicinity of the stimulation sites. In contrast, in networks with longer synaptic length scales, the spatiotemporal sequence of stimulus deliveries is of major importance for synaptic downregulation. In particular, rapid shuffling of the stimulus sequence is advantageous for synaptic downregulation.
Conclusion: Our results suggest that CR stimulation parameters can be adjusted to synaptic connectivity to further improve the long-lasting effects. Furthermore, shuffling of CR sequences is advantageous for long-lasting desynchronization effects. Our work provides important hypotheses on CR parameter selection for future preclinical and clinical studies.
Keywords: coordinated reset stimulation; desynchronization; networks of spiking neurons; spatial neural networks; spike-timing-dependent plasticity; synchronization.
Copyright © 2024 Kromer and Tass.
Conflict of interest statement
JK and PT filed a Stanford-owned provisional patent related to the presented results. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






Similar articles
-
Long-Lasting Desynchronization of Plastic Neuronal Networks by Double-Random Coordinated Reset Stimulation.Front Netw Physiol. 2022 Apr 19;2:864859. doi: 10.3389/fnetp.2022.864859. eCollection 2022. Front Netw Physiol. 2022. PMID: 36926109 Free PMC article.
-
Long-Lasting Desynchronization Effects of Coordinated Reset Stimulation Improved by Random Jitters.Front Physiol. 2021 Sep 24;12:719680. doi: 10.3389/fphys.2021.719680. eCollection 2021. Front Physiol. 2021. PMID: 34630142 Free PMC article.
-
Simulated dataset on coordinated reset stimulation of homogeneous and inhomogeneous networks of excitatory leaky integrate-and-fire neurons with spike-timing-dependent plasticity.Data Brief. 2024 Mar 20;54:110345. doi: 10.1016/j.dib.2024.110345. eCollection 2024 Jun. Data Brief. 2024. PMID: 38586130 Free PMC article.
-
Maladaptive neural synchrony in tinnitus: origin and restoration.Front Neurol. 2015 Feb 17;6:29. doi: 10.3389/fneur.2015.00029. eCollection 2015. Front Neurol. 2015. PMID: 25741316 Free PMC article. Review.
-
Spike-Timing-Dependent Plasticity Mediated by Dopamine and its Role in Parkinson's Disease Pathophysiology.Front Netw Physiol. 2022 Mar 4;2:817524. doi: 10.3389/fnetp.2022.817524. eCollection 2022. Front Netw Physiol. 2022. PMID: 36926058 Free PMC article. Review.
Cited by
-
Amplifying post-stimulation oscillatory dynamics by engaging synaptic plasticity with transcranial alternating current stimulation.Front Netw Physiol. 2025 Jul 18;5:1621283. doi: 10.3389/fnetp.2025.1621283. eCollection 2025. Front Netw Physiol. 2025. PMID: 40756655 Free PMC article.
-
Data-driven and equation-free methods for neurological disorders: analysis and control of the striatum network.Front Netw Physiol. 2024 Aug 7;4:1399347. doi: 10.3389/fnetp.2024.1399347. eCollection 2024. Front Netw Physiol. 2024. PMID: 39171120 Free PMC article.
References
LinkOut - more resources
Full Text Sources

