Identification of Mycoplasma pneumoniae proteins interacting with NOD2 and their role in macrophage inflammatory response
- PMID: 38863748
- PMCID: PMC11165193
- DOI: 10.3389/fmicb.2024.1391453
Identification of Mycoplasma pneumoniae proteins interacting with NOD2 and their role in macrophage inflammatory response
Abstract
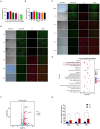
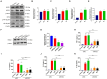
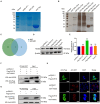
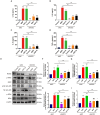
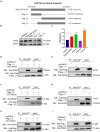
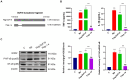
Mycoplasma pneumoniae (M. pneumoniae, Mp) is a cell wall-deficient microorganism known to cause chronic respiratory infections in both children and adults. Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is an intracellular pattern recognition receptor primarily responsible for identifying muramyl dipeptide (MDP) found in bacterial cell walls. Previous experiments have demonstrated that Mycoplasma ovipneumoniae induces macrophage autophagy through NOD2. In this study, we conducted RNA-seq analysis on macrophages infected with M. pneumoniae and observed an up-regulation in the expression of genes associated with the NOD2 signaling pathway. Mechanistic investigations further revealed the involvement of the NOD2 signaling pathway in the inflammatory response of macrophages activated by M. pneumoniae. We utilized GST pull-down technology in conjunction with liquid chromatography-tandem mass spectrometry (LC-MS/MS) to pinpoint the M. pneumoniae proteins that interact with NOD2. Additionally, co-immunoprecipitation (Co-IP) and immunofluorescence co-localization techniques were used to confirm the interaction between DUF16 protein and NOD2. We found that DUF16 protein can enter macrophages and induce macrophage inflammatory response through the NOD2/RIP2/NF-κB pathway. Notably, the region spanning amino acids 13-90 was identified as a critical region necessary for DUF16-induced inflammation. This research not only broadens our comprehension of the recognition process of the intracellular receptor NOD2, but also deepens our understanding of the development of M. pneumoniae infection.
Keywords: DUF16; Mycoplasma pneumoniae; NF-κB; NOD2; inflammation; macrophages.
Copyright © 2024 Wang, Ma, Hao, Wang, Luo and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
NOD2/c-Jun NH2-Terminal Kinase Triggers Mycoplasma ovipneumoniae-Induced Macrophage Autophagy.J Bacteriol. 2020 Sep 23;202(20):e00689-19. doi: 10.1128/JB.00689-19. Print 2020 Sep 23. J Bacteriol. 2020. PMID: 32778560 Free PMC article.
-
Hepatic NOD2 promotes hepatocarcinogenesis via a RIP2-mediated proinflammatory response and a novel nuclear autophagy-mediated DNA damage mechanism.J Hematol Oncol. 2021 Jan 7;14(1):9. doi: 10.1186/s13045-020-01028-4. J Hematol Oncol. 2021. PMID: 33413510 Free PMC article.
-
Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae.J Biol Chem. 2004 Aug 27;279(35):36426-32. doi: 10.1074/jbc.M403861200. Epub 2004 Jun 23. J Biol Chem. 2004. PMID: 15215247
-
Nucleotide-binding oligomerization domain containing 2: structure, function, and diseases.Semin Arthritis Rheum. 2013 Aug;43(1):125-30. doi: 10.1016/j.semarthrit.2012.12.005. Epub 2013 Jan 24. Semin Arthritis Rheum. 2013. PMID: 23352252 Review.
-
Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation.Cell Microbiol. 2003 Sep;5(9):581-92. doi: 10.1046/j.1462-5822.2003.00304.x. Cell Microbiol. 2003. PMID: 12925128 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

