Swietemicrolides A-D, mexicanolide-type limonoids from the bark of Swietenia macrophylla with in vitro cytotoxic and α-glucosidase inhibitory activities
- PMID: 38863811
- PMCID: PMC11165692
- DOI: 10.1039/d4ra01954g
Swietemicrolides A-D, mexicanolide-type limonoids from the bark of Swietenia macrophylla with in vitro cytotoxic and α-glucosidase inhibitory activities
Abstract
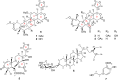
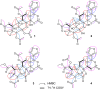
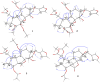
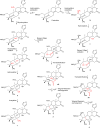
Four new mexicanolide-type limonoids, swietemicrolides A-D (1-4), together with three known compounds (5-7) were isolated from an ethyl acetate extract of the bark of Swietenia microphylla. 1 and 2 had 1,8-hemiacetal systems whilst 3 and 4 shared hexacyclic skeletons consisting of three fused five-membered rings. The structures of the isolated compounds were determined using spectroscopic methods. The five limonoids (1-5) were tested in vitro for their cytotoxic effects against two human cancer cell lines (KB carcinoma and A549 lung cancer cells) and α-glucosidase inhibitory activity. None of them showed significant cytotoxic activity, however, swietemicrolide C (3) exhibited strong effect towards α-glucosidase. Moreover, a possible biosynthetic pathway for compounds 1-4 was proposed to support a comprehensive understanding of the configurations of the new limonoids.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Limonoids from the seeds of Swietenia macrophylla with inhibitory activity against dengue virus 2.J Nat Prod. 2014 Nov 26;77(11):2367-74. doi: 10.1021/np5002829. Epub 2014 Oct 20. J Nat Prod. 2014. PMID: 25330401
-
Limonoids and triterpenoid from fruit of Swietenia macrophylla.Fitoterapia. 2018 Mar;125:141-146. doi: 10.1016/j.fitote.2018.01.004. Epub 2018 Jan 8. Fitoterapia. 2018. PMID: 29325928
-
Mexicanolide-type limonoids from the seeds of Swietenia macrophylla.J Asian Nat Prod Res. 2018 Apr;20(4):299-305. doi: 10.1080/10286020.2017.1335715. Epub 2017 Jun 1. J Asian Nat Prod Res. 2018. PMID: 28569087
-
The recent use of Swietenia mahagoni (L.) Jacq. as antidiabetes type 2 phytomedicine: A systematic review.Heliyon. 2020 Mar 10;6(3):e03536. doi: 10.1016/j.heliyon.2020.e03536. eCollection 2020 Mar. Heliyon. 2020. PMID: 32190758 Free PMC article. Review.
-
Chemical Structures and Biological Activities of Limonoids from the Genus Swietenia (Meliaceae).Molecules. 2018 Jun 29;23(7):1588. doi: 10.3390/molecules23071588. Molecules. 2018. PMID: 29966275 Free PMC article. Review.
Cited by
-
Toxicity and Metabolomic Dysfunction Invoked by Febrifugin, a Harmful Component of Edible Nut of Swietenia macrophylla.Int J Mol Sci. 2024 Sep 9;25(17):9753. doi: 10.3390/ijms25179753. Int J Mol Sci. 2024. PMID: 39273700 Free PMC article.
References
-
- Cheng Y. B. Chien Y. T. Lee J. C. Tseng C. K. Wang H. C. Lo I. W. Wu Y. H. Wang S. Y. Wu Y. C. Chang F. R. J. Nat. Prod. 2014;77:2367–2374. - PubMed
-
- Abdelgaleil S. A. Doe M. Morimoto Y. Nakatani M. Phytochemistry. 2006;67:452–458. - PubMed
-
- Fowles R. Mootoo B. Ramsewak R. Khan A. Ramsubhag A. Reynolds W. Nair M. Pest Manag. Sci. 2010;66:1298–1303. - PubMed
-
- Mootoo B. S. Ali A. Motilal R. Pingal R. Ramlal A. Khan A. Reynolds W. F. McLean S. J. Nat. Prod. 1999;62:1514–1517. - PubMed
LinkOut - more resources
Full Text Sources