Simple and direct electrochemical detection of rosmarinic acid in food samples based on nanochannel modified carbon electrode
- PMID: 38863812
- PMCID: PMC11165691
- DOI: 10.1039/d4ra03063j
Simple and direct electrochemical detection of rosmarinic acid in food samples based on nanochannel modified carbon electrode
Abstract
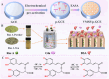
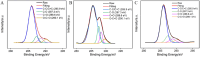
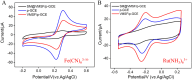
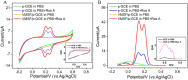
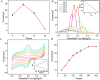
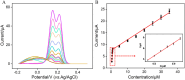
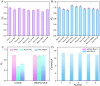
The detection of rosmarinic acid (Ros A) in food samples holds major significance. Simple and convenient electrochemical detection of Ros A with high performance remains a challenge. In this work, a nanochannel array-modified carbon electrode was constructed using a simple and convenient approach to achieve highly sensitive electrochemical detection of Ros A in food samples. Through simple electrochemical pre-activation of a glassy carbon electrode (GCE), oxygen-containing functional groups were introduced on the electrode surface (p-GCE). Vertically-ordered mesoporous silica film (VMSF) was stably grown on p-GCE through electrochemical-assisted self-assembly (EASA) without the introduction of another adhesive layer (VMSF/p-GCE). Transmission electron microscopy (TEM) characterization demonstrated the highly ordered structure of VMSF with a nanochannel diameter around 2.7 nm. Both p-GCE and the nanochannels significantly enhanced the electrochemical signals of Ros A on the electrode, exhibiting dual signal amplification. VMSF/p-GCE demonstrated sensitive detection of Ros A with a linear range of 500 nM to 1 μM and 1 μM to 35 μM. The detection limit (DL) was 26 nM. Combining the good anti-fouling and anti-interference properties of the nanochannels, VMSF/p-GCE can achieve direct electrochemical detection of Ros A in food samples. The sensor can be easily regenerated for repeated use. The simple fabrication, high detection sensitivity and selectivity of the sensor make it a new strategy for rapid preparation of high-performance electrochemical sensors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Simple fabrication of electrochemical sensor based on integration of dual signal amplification by the supporting electrode and modified nanochannel array for direct and sensitive detection of vitamin B2.Front Nutr. 2024 Mar 15;11:1352938. doi: 10.3389/fnut.2024.1352938. eCollection 2024. Front Nutr. 2024. PMID: 38559779 Free PMC article.
-
Sensitive electrochemical detection of p-nitrophenol by pre-activated glassy carbon electrode integrated with silica nanochannel array film.Front Chem. 2022 Aug 5;10:954748. doi: 10.3389/fchem.2022.954748. eCollection 2022. Front Chem. 2022. PMID: 35991606 Free PMC article.
-
Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes.Front Chem. 2023 Feb 15;11:1126213. doi: 10.3389/fchem.2023.1126213. eCollection 2023. Front Chem. 2023. PMID: 36874060 Free PMC article.
-
Integration of vertically-ordered mesoporous silica-nanochannel film with electro-activated glassy carbon electrode for improved electroanalysis in complex samples.Talanta. 2021 Apr 1;225:122066. doi: 10.1016/j.talanta.2020.122066. Epub 2020 Dec 29. Talanta. 2021. PMID: 33592785
-
Solid-state nanochannels based on electro-optical dual signals for detection of analytes.Talanta. 2024 Nov 1;279:126615. doi: 10.1016/j.talanta.2024.126615. Epub 2024 Aug 2. Talanta. 2024. PMID: 39096787 Review.
Cited by
-
Graphitic carbon nitride nanosheet supported silica nanochannel film for enhanced electrochemiluminescence sensing of 2,4,6-trichlorophenol and prochloraz.RSC Adv. 2024 Sep 12;14(39):28976-28983. doi: 10.1039/d4ra03623a. eCollection 2024 Sep 4. RSC Adv. 2024. PMID: 39268050 Free PMC article.
References
LinkOut - more resources
Full Text Sources

