CRISPR-Cas9 immune-evasive hESCs are rejected following transplantation into immunocompetent mice
- PMID: 38863835
- PMCID: PMC11165197
- DOI: 10.3389/fgeed.2024.1403395
CRISPR-Cas9 immune-evasive hESCs are rejected following transplantation into immunocompetent mice
Abstract
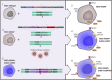
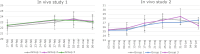
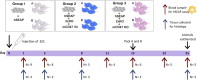
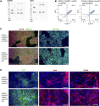
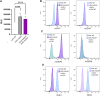
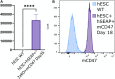
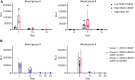
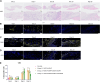
Although current stem cell therapies exhibit promising potential, the extended process of employing autologous cells and the necessity for donor-host matching to avert the rejection of transplanted cells significantly limit the widespread applicability of these treatments. It would be highly advantageous to generate a pluripotent universal donor stem cell line that is immune-evasive and, therefore, not restricted by the individual's immune system, enabling unlimited application within cell replacement therapies. Before such immune-evasive stem cells can be moved forward to clinical trials, in vivo testing via transplantation experiments in immune-competent animals would be a favorable approach preceding preclinical testing. By using human stem cells in immune competent animals, results will be more translatable to a clinical setting, as no parts of the immune system have been altered, although in a xenogeneic setting. In this way, immune evasiveness, cell survival, and unwanted proliferative effects can be assessed before clinical trials in humans. The current study presents the generation and characterization of three human embryonic stem cell lines (hESCs) for xenogeneic transplantation in immune-competent mice. The major histocompatibility complexes I- and II-encoding genes, B2M and CIITA, have been deleted from the hESCs using CRISPR-Cas9-targeted gene replacement strategies and knockout. B2M was knocked out by the insertion of murine CD47. Human-secreted embryonic alkaline phosphatase (hSEAP) was inserted in a safe harbor site to track cells in vivo. The edited hESCs maintained their pluripotency, karyotypic normality, and stable expression of murine CD47 and hSEAP in vitro. In vivo transplantation of hESCs into immune-competent BALB/c mice was successfully monitored by measuring hSEAP in blood samples. Nevertheless, transplantation of immune-evasive hESCs resulted in complete rejection within 11 days, with clear immune infiltration of T-cells on day 8. Our results reveal that knockout of B2M and CIITA together with species-specific expression of CD47 are insufficient to prevent rejection in an immune-competent and xenogeneic context.
Keywords: CRISPR-Cas9 editing; human embryonic stem cells; immune rejection; transgene insertion; universal cell line; xenogeneic transplantation.
Copyright © 2024 Frederiksen, Glantz, Vøls, Skov, Tveden-Nyborg, Freude and Doehn.
Conflict of interest statement
Authors AG, KV and UD were employed by Novo Nordisk A/S. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Targeted Disruption of the β2-Microglobulin Gene Minimizes the Immunogenicity of Human Embryonic Stem Cells.Stem Cells Transl Med. 2015 Oct;4(10):1234-45. doi: 10.5966/sctm.2015-0049. Epub 2015 Aug 18. Stem Cells Transl Med. 2015. PMID: 26285657 Free PMC article.
-
Development of a baculoviral CRISPR/Cas9 vector system for beta-2-microglobulin knockout in human pluripotent stem cells.Mol Genet Genomics. 2024 Aug 1;299(1):74. doi: 10.1007/s00438-024-02167-w. Mol Genet Genomics. 2024. PMID: 39085666
-
Novel traceable CRISPR-Cas9 engineered human embryonic stem cell line (E1C3 + hSEAP + 2xKO + pCD47), has potential to evade immune detection in pigs.Stem Cell Res. 2024 Jun;77:103438. doi: 10.1016/j.scr.2024.103438. Epub 2024 May 7. Stem Cell Res. 2024. PMID: 38776701
-
Human embryonic stem cells: potential tool for achieving immunotolerance?Stem Cell Rev. 2005;1(2):151-8. doi: 10.1385/SCR:1:2:151. Stem Cell Rev. 2005. PMID: 17142850 Review.
-
Universal cell donor lines: A review of the current research.Stem Cell Reports. 2023 Nov 14;18(11):2038-2046. doi: 10.1016/j.stemcr.2023.09.010. Epub 2023 Oct 12. Stem Cell Reports. 2023. PMID: 37832541 Free PMC article. Review.
Cited by
-
Role and Mechanism of Olfactory Stem Cells in the Treatment of Olfactory Disorders.Stem Cells Int. 2025 Apr 24;2025:6631857. doi: 10.1155/sci/6631857. eCollection 2025. Stem Cells Int. 2025. PMID: 40313858 Free PMC article. Review.
-
Induced Pluripotent Stem Cells-Based Regenerative Therapies in Treating Human Aging-Related Functional Decline and Diseases.Cells. 2025 Apr 21;14(8):619. doi: 10.3390/cells14080619. Cells. 2025. PMID: 40277944 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

