Clinical Outcomes of Intravenous Methylnaltrexone in Children: A Single-Arm Retrospective Cohort Study
- PMID: 38863861
- PMCID: PMC11163904
- DOI: 10.5863/1551-6776-29.3.292
Clinical Outcomes of Intravenous Methylnaltrexone in Children: A Single-Arm Retrospective Cohort Study
Abstract
Objectives: Constipation is a common adverse event of opioid use that is often difficult to treat. Methylnaltrexone is a therapeutic option for opioid-induced constipation (OIC) approved for oral and subcutaneous use in adults. These administration routes are not always feasible in the pediatric population. The primary objective of this research was to quantify the response rate of methylnaltrexone in pediatric patients when it was administered via the intravenous (IV) route.
Methods: This retrospective study evaluated patients ages <18 years who received IV methylnaltrexone between January 1, 2013, and June 30, 2020, for OIC. Efficacy was evaluated through documentation of bowel evacuation within 4 hours of methylnaltrexone administration. Adverse events observed within 24 hours of administration were attributed to methylnaltrexone.
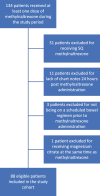
Results: Methylnaltrexone was administered to 134 unique patients during the study period. Of these, 46 met exclusion criteria, resulting in 88 patients being included in the study. Patients with an underlying hematology/oncology diagnosis consisted of 77% of the study population, and 23% of patients had an -underlying medical/surgical diagnosis. The response rate to IV methylnaltrexone was 25% (CI, 16-34).
Conclusions: The results of this retrospective chart review demonstrate the potential role of IV methylnaltrexone in the pediatric population. Despite the overall lower response rate relative to that reported in adults, IV methylnaltrexone possesses a unique mechanism of action that may serve as an alternative treatment option for patients unable to use the oral and subcutaneous administration routes. There were no significant adverse events seen in the study.
Keywords: constipation; intravenous; methylnaltrexone; oncology; opioid-induced constipation; pediatrics.
Copyright. Pediatric Pharmacy Association. All rights reserved. For permissions, email: membership@pediatricpharmacy.org.
Conflict of interest statement
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. MR and DW had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
Similar articles
-
Successful Use of Intravenous Methylnaltrexone for Opioid-Induced Constipation in Critically Ill Pediatric Patients.J Pediatr Intensive Care. 2021 Oct 7;13(1):25-31. doi: 10.1055/s-0041-1736335. eCollection 2024 Mar. J Pediatr Intensive Care. 2021. PMID: 38571990 Free PMC article.
-
The safety and efficacy of methylnaltrexone in pediatric oncology patients: A single center experience.J Oncol Pharm Pract. 2024 Jan;30(1):4-8. doi: 10.1177/10781552231163540. Epub 2023 Mar 22. J Oncol Pharm Pract. 2024. PMID: 36946143
-
Methylnaltrexone: MNTX.Drugs R D. 2006;7(6):374-8. doi: 10.2165/00126839-200607060-00006. Drugs R D. 2006. PMID: 17073520 Review.
-
Methylnaltrexone for opioid-induced constipation in pediatric oncology patients.Pediatr Blood Cancer. 2013 Oct;60(10):1667-70. doi: 10.1002/pbc.24615. Epub 2013 Jun 14. Pediatr Blood Cancer. 2013. PMID: 23766091 Clinical Trial.
-
Methylnaltrexone bromide for the treatment of opioid-induced constipation.Expert Opin Pharmacother. 2018 Jul;19(10):1127-1135. doi: 10.1080/14656566.2018.1491549. Epub 2018 Jul 6. Expert Opin Pharmacother. 2018. PMID: 29979903 Review.
Cited by
-
Naldemedine for the management of opioid-induced constipation in patients with cancer pain: A narrative review.Medicine (Baltimore). 2025 Aug 1;104(31):e43644. doi: 10.1097/MD.0000000000043644. Medicine (Baltimore). 2025. PMID: 40760572 Free PMC article. Review.
References
-
- Holzer P. Methylnaltrexone for the management of unwanted peripheral opioid effects. Therapy . 2008;5(4):531–543.
-
- Methylnaltrexone Lexi-Drugs. Accessed August 23, 2020. http://online.lexi.com.
-
- Relistor [package insert] Bridgewater, NJ: Salix Pharmaceuticals; 2020.
-
- Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain . 2011;12(5):554–562. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous