Thermotolerance Mechanism of Fungal GH6 Cellobiohydrolase. Part II. Structural Analysis of Thermotolerant Mutant from the Basidiomycete Phanerochaete chrysosporium
- PMID: 38863950
- PMCID: PMC11163327
- DOI: 10.5458/jag.jag.JAG-2023_0018
Thermotolerance Mechanism of Fungal GH6 Cellobiohydrolase. Part II. Structural Analysis of Thermotolerant Mutant from the Basidiomycete Phanerochaete chrysosporium
Abstract
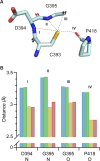
Glycoside hydrolase family 6 cellobiohydrolase (GH6 CBH) is a group of cellulases capable of hydrolyzing crystalline cellulose. However, the synergistic reaction of GH6 CBH with other cellulases is hindered by its relatively low thermotolerance. We previously obtained a thermotolerant double mutant, C240S/C393S, of GH6 CBH from the basidiomycete Phanerochaete chrysosporium (PcCel6A) by replacing the two free cysteine (Cys) residues, C240 and C393, with serine (Yamaguchi et al., J Appl Glycosci. 2020; 67;79-86). In the accompanying paper (Part I; Yamaguchi et al., J Appl Glycosci. 2024; 71: 55-62), we measured the temperature dependence of the activity and folding of C240S/C393S and its single mutants, C240S and C393S, and found that replacement of C393 was the major contributor to the increased thermotolerance of C240S/C393S. Here, in order to investigate the mechanism involved, we crystallized the wild-type and the mutant enzymes and compared their X-ray crystal structures. The overall structures of the wild-type and the three mutant enzymes were similar. However, C240S/C393S had the lowest relative B-factor at both the N-terminal loop (residues 172-177) and the C-terminal loop (residues 390-425). This result suggests that reduced structural fluctuation of the substrate-enclosing loops, possibly due to stronger hydrogen bonding involving C393, could account for the increased thermotolerance of C240S/C393S.
Keywords: Cellulose; GH6; X-ray crystal structure; cellobiohydrolase; free cysteine; thermotolerance.
2024 by The Japanese Society of Applied Glycoscience.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Thermotolerance Mechanism of Fungal GH6 Cellobiohydrolase. Part I. Characterization of Thermotolerant Mutant from the Basidiomycete Phanerochaete chrysosporium.J Appl Glycosci (1999). 2024 May 20;71(2):55-62. doi: 10.5458/jag.jag.JAG-2023_0017. eCollection 2024. J Appl Glycosci (1999). 2024. PMID: 38863951 Free PMC article.
-
Crystal structure of a family 6 cellobiohydrolase from the basidiomycete Phanerochaete chrysosporium.Acta Crystallogr F Struct Biol Commun. 2017 Jul 1;73(Pt 7):398-403. doi: 10.1107/S2053230X17008093. Epub 2017 Jun 17. Acta Crystallogr F Struct Biol Commun. 2017. PMID: 28695848 Free PMC article.
-
Loop motions important to product expulsion in the Thermobifida fusca glycoside hydrolase family 6 cellobiohydrolase from structural and computational studies.J Biol Chem. 2013 Nov 15;288(46):33107-17. doi: 10.1074/jbc.M113.502765. Epub 2013 Sep 30. J Biol Chem. 2013. PMID: 24085303 Free PMC article.
-
The structure of a bacterial cellobiohydrolase: the catalytic core of the Thermobifida fusca family GH6 cellobiohydrolase Cel6B.J Mol Biol. 2013 Feb 8;425(3):622-35. doi: 10.1016/j.jmb.2012.11.039. Epub 2012 Dec 5. J Mol Biol. 2013. PMID: 23220193
-
A novel GH6 cellobiohydrolase from Paenibacillus curdlanolyticus B-6 and its synergistic action on cellulose degradation.Appl Microbiol Biotechnol. 2017 Feb;101(3):1175-1188. doi: 10.1007/s00253-016-7895-8. Epub 2016 Oct 14. Appl Microbiol Biotechnol. 2017. PMID: 27743043
References
-
- Boisset C, Fraschini C, Schülein M, Henrissat B, Chanzy H. Imaging the enzymatic digestion of bacterial cellulose ribbons reveals the endo character of the cellobiohydrolase Cel6A from Humicola insolens and its mode of synergy with cellobiohydrolase Cel7A. Appl Environ Microbiol. 2000; 66: 1444-52. - PMC - PubMed
-
- Abuja PM, Pilz I, Claessens M, Tomme P. Domain structure of cellobiohydrolase II as studied by small angle X-ray scattering: close resemblance to cellobiohydrolase I. Biochem Biophys Res Commun. 1988; 156: 180-5. - PubMed
-
- Igarashi K, Uchihashi T, Koivula A, Wada M, Kimura S, Okamoto T, et al. Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Science. 2011; 333: 1279-82. - PubMed
LinkOut - more resources
Full Text Sources
