Renal Tissue-Derived Exosomal miRNA-34a in Diabetic Nephropathy Induces Renal Tubular Cell Fibrosis by Promoting the Polarization of M1 Macrophages
- PMID: 38863972
- PMCID: PMC11095076
- DOI: 10.1049/2024/5702517
Renal Tissue-Derived Exosomal miRNA-34a in Diabetic Nephropathy Induces Renal Tubular Cell Fibrosis by Promoting the Polarization of M1 Macrophages
Abstract
Background: Diabetic nephropathy (DN) is the leading cause of chronic kidney disease, and the activation and infiltration of phagocytes are critical steps of DN. This study aimed to explore the mechanism of exosomes in macrophages and diabetes nephropathy and the role of miRNA-34a, which might provide a new path for treating DN.
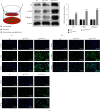
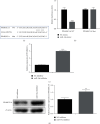
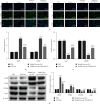
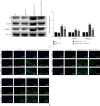
Materials and methods: The DN model was established, and the success of the model establishment was confirmed by detecting general indicators, HE staining, and immunohistochemistry. Electron microscopy and NanoSight Tracking Analysis (NTA) were used to see the morphology and size of exosomes. MiRNA-34a inhibitor, miRNA-34a mimics, pc-PPARGC1A, and controls were transfected in macrophages with or without kidney exosomal. A dual-luciferase reporter gene experiment verifies the targeting relationship between miRNA-34a and PPARGC1A. After exosomal culture, macrophages are co-cultured with normal renal tubular cells to detect renal tubular cell fibrosis. Q-PCR and western blot were undertaken to detect related RNA and proteins.
Results: An animal model of diabetic nephropathy was successfully constructed. Macrophages could phagocytose exosomes. After ingesting model exosomes, M1 macrophages were activated, while M2 macrophages were weakened, indicating the model mice's kidney exosomes caused the polarization. MiRNA-34a inhibitor increased PPARGC1A expression. MiRNA-34a expressed higher in diabetic nephropathy Model-Exo. MiRNA-34a negatively regulated PPARGC1A. PPARGC1A rescued macrophage polarization and renal tubular cell fibrosis.
Conclusion: Exosomal miRNA-34a of tubular epithelial cells promoted M1 macrophage activation in diabetic nephropathy via negatively regulating PPARGC1A expression, which may provide a new direction for further exploration of DN treatment.
Copyright © 2024 Shuai Zheng et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Macrophage-derived exosomes promote telomere fragility and senescence in tubular epithelial cells by delivering miR-155.Cell Commun Signal. 2024 Jul 10;22(1):357. doi: 10.1186/s12964-024-01708-5. Cell Commun Signal. 2024. PMID: 38987851 Free PMC article.
-
Circulating exosome-circRNAs mediated downregulation of FGF9 through ceRNA mechanism aggravates renal fibrosis in diabetic nephropathy.PLoS One. 2025 Jun 17;20(6):e0326217. doi: 10.1371/journal.pone.0326217. eCollection 2025. PLoS One. 2025. PMID: 40526701 Free PMC article.
-
The axis of miR-30a/AIF-1/TRPC6/calcineurin A/NFAT2 regulated the death modalities and inflammation of renal tubular epithelial cells in diabetic kidney disease via exosome.Life Sci. 2025 Sep 15;377:123760. doi: 10.1016/j.lfs.2025.123760. Epub 2025 May 24. Life Sci. 2025. PMID: 40419106
-
Research progress on non-coding RNA regulatory networks and targeted therapy in diabetic nephropathy.Front Endocrinol (Lausanne). 2025 Jul 29;16:1625307. doi: 10.3389/fendo.2025.1625307. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40801037 Free PMC article. Review.
-
Endothelial nitric oxide synthase gene polymorphisms and risk of diabetic nephropathy: a systematic review and meta-analysis.BMC Med Genet. 2014 Jan 16;15:9. doi: 10.1186/1471-2350-15-9. BMC Med Genet. 2014. PMID: 24433471 Free PMC article.
Cited by
-
Exosomes and Macrophages: Bidirectional Mutual Regulation in the Treatment of Diabetic Complications.Cell Mol Bioeng. 2024 Aug 31;17(4):243-261. doi: 10.1007/s12195-024-00816-z. eCollection 2024 Aug. Cell Mol Bioeng. 2024. PMID: 39372550 Free PMC article. Review.
-
Exo-hydrogel therapy: a revolutionary approach to managing diabetic complications.J Nanobiotechnology. 2025 Aug 11;23(1):558. doi: 10.1186/s12951-025-03621-6. J Nanobiotechnology. 2025. PMID: 40790200 Free PMC article. Review.
-
Extracellular Vesicles in Renal Inflammatory Diseases: Revealing Mechanisms of Extracellular Vesicle-Mediated Macrophage Regulation.Int J Mol Sci. 2025 Apr 12;26(8):3646. doi: 10.3390/ijms26083646. Int J Mol Sci. 2025. PMID: 40332144 Free PMC article. Review.
-
Frontier role of extracellular vesicles in kidney disease.J Nanobiotechnology. 2024 Sep 20;22(1):583. doi: 10.1186/s12951-024-02852-3. J Nanobiotechnology. 2024. PMID: 39304945 Free PMC article. Review.
-
Extracellular vesicle-based drug overview: research landscape, quality control and nonclinical evaluation strategies.Signal Transduct Target Ther. 2025 Aug 14;10(1):255. doi: 10.1038/s41392-025-02312-w. Signal Transduct Target Ther. 2025. PMID: 40804047 Free PMC article. Review.
References
-
- Yang L. Adriamycin nephrotoxicity is reduced by metallothionein over-expression and kidney gene expression is modified by diabetes in the OVE26 diabetic model. Electronic Theses and Dissertations . 2010 doi: 10.18297/etd/1618. - DOI
-
- Gao H. X., Regier E., Close K. L. International diabetes federation world diabetes congress 2015 (IDF 2015) Journal of Diabetes . 2016;8(3) - PubMed
-
- Liu Y., Jia J., Lin S. Renal pathological changes and autophagy-related protein expression in diabetic nephropathy rats after intraperitoneal injection of paricalcitol. Shandong Medical Journal . 2019;59(24):48–51.
-
- Hirata M., Muramoto H., Haruki K., Tofuku Y., Takeda R. [Impaired metabolism of guanidinoacetic acid in uremia, with special reference to diabetic nephropathy] Nihon Jinzo Gakkai Shi . 1988;30(2):129–136. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

