Integrated transcriptomic and immunological profiling reveals new diagnostic and prognostic models for cutaneous melanoma
- PMID: 38863979
- PMCID: PMC11165152
- DOI: 10.3389/fphar.2024.1389550
Integrated transcriptomic and immunological profiling reveals new diagnostic and prognostic models for cutaneous melanoma
Abstract
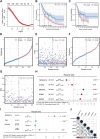
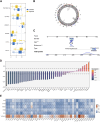
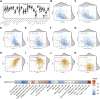
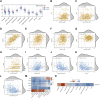
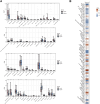
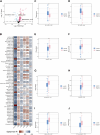
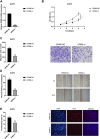
The mortality rate associated with cutaneous melanoma (SKCM) remains alarmingly high, highlighting the urgent need for a deeper understanding of its molecular underpinnings. In our study, we leveraged bulk transcriptome sequencing data from the SKCM cohort available in public databases such as TCGA and GEO. We utilized distinct datasets for training and validation purposes and also incorporated mutation and clinical data from TCGA, along with single-cell sequencing data from GEO. Through dimensionality reduction, we annotated cell subtypes within the single-cell data and analyzed the expression of tumor-related pathways across these subtypes. We identified differentially expressed genes (DEGs) in the training set, which were further refined using the Least Absolute Shrinkage and Selection Operator (LASSO) machine learning algorithm, employing tenfold cross-validation. This enabled the construction of a prognostic model, whose diagnostic efficacy we subsequently validated. We conducted Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses on the DEGs, and performed immunological profiling on two risk groups to elucidate the relationship between model genes and the immune responses relevant to SKCM diagnosis, treatment, and prognosis. We also knocked down the GMR6 expression level in the melanoma cells and verified its effect on cancer through multiple experiments. The results indicate that the GMR6 gene plays a role in promoting the proliferation, invasion, and migration of cancer cells in human melanoma. Our findings offer novel insights and a theoretical framework that could enhance prognosis, treatment, and drug development strategies for SKCM, potentially leading to more precise therapeutic interventions.
Keywords: LASSO machine learning algorithm; SKCM; differential gene expression; immune infiltration analysis; melanoma.
Copyright © 2024 Li, Wu, Lin, Zhou and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Identification of anoikis-related subtypes and development of risk stratification system in skin cutaneous melanoma.Heliyon. 2023 May 9;9(5):e16153. doi: 10.1016/j.heliyon.2023.e16153. eCollection 2023 May. Heliyon. 2023. PMID: 37215879 Free PMC article.
-
Analysis on tumor immune microenvironment and construction of a prognosis model for immune-related skin cutaneous melanoma.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 May 28;48(5):671-681. doi: 10.11817/j.issn.1672-7347.2023.230069. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37539569 Free PMC article. Chinese, English.
-
HOPX is a tumor-suppressive biomarker that corresponds to T cell infiltration in skin cutaneous melanoma.Cancer Cell Int. 2023 Jun 21;23(1):122. doi: 10.1186/s12935-023-02962-2. Cancer Cell Int. 2023. PMID: 37344870 Free PMC article.
-
Construction and validation of a novel pyroptosis-related signature to predict prognosis in patients with cutaneous melanoma.Math Biosci Eng. 2022 Jan;19(1):688-706. doi: 10.3934/mbe.2022031. Epub 2021 Nov 19. Math Biosci Eng. 2022. PMID: 34903008
-
The Landscape of the Tumor Microenvironment in Skin Cutaneous Melanoma Reveals a Prognostic and Immunotherapeutically Relevant Gene Signature.Front Cell Dev Biol. 2021 Oct 1;9:739594. doi: 10.3389/fcell.2021.739594. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34660598 Free PMC article.
Cited by
-
Cutaneous Melanoma: An Overview of Physiological and Therapeutic Aspects and Biotechnological Use of Serine Protease Inhibitors.Molecules. 2024 Aug 16;29(16):3891. doi: 10.3390/molecules29163891. Molecules. 2024. PMID: 39202970 Free PMC article. Review.
References
-
- Dhingra A., Fina M. E., Neinstein A., Ramsey D. J., Xu Y., Fishman G. A., et al. (2011). Autoantibodies in melanoma-associated retinopathy target TRPM1 cation channels of retinal ON bipolar cells. J. Neurosci. official J. Soc. Neurosci. 31 (11), 3962–3967. 10.1523/JNEUROSCI.6007-10.2011 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

