Incidence and management of the main serious adverse events reported after COVID-19 vaccination
- PMID: 38864106
- PMCID: PMC11167235
- DOI: 10.1002/prp2.1224
Incidence and management of the main serious adverse events reported after COVID-19 vaccination
Abstract
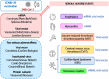
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2n first appeared in Wuhan, China in 2019. Soon after, it was declared a pandemic by the World Health Organization. The health crisis imposed by a new virus and its rapid spread worldwide prompted the fast development of vaccines. For the first time in human history, two vaccines based on recombinant genetic material technology were approved for human use. These mRNA vaccines were applied in massive immunization programs around the world, followed by other vaccines based on more traditional approaches. Even though all vaccines were tested in clinical trials prior to their general administration, serious adverse events, usually of very low incidence, were mostly identified after application of millions of doses. Establishing a direct correlation (the cause-effect paradigm) between vaccination and the appearance of adverse effects has proven challenging. This review focuses on the main adverse effects observed after vaccination, including anaphylaxis, myocarditis, vaccine-induced thrombotic thrombocytopenia, Guillain-Barré syndrome, and transverse myelitis reported in the context of COVID-19 vaccination. We highlight the symptoms, laboratory tests required for an adequate diagnosis, and briefly outline the recommended treatments for these adverse effects. The aim of this work is to increase awareness among healthcare personnel about the serious adverse events that may arise post-vaccination. Regardless of the ongoing discussion about the safety of COVID-19 vaccination, these adverse effects must be identified promptly and treated effectively to reduce the risk of complications.
Keywords: COVID‐19; SARS‐CoV‐2; adverse effects; vaccines.
© 2024 The Author(s). Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Background incidence rates of adverse events of special interest related to COVID-19 vaccines in Ontario, Canada, 2015 to 2020, to inform COVID-19 vaccine safety surveillance.Vaccine. 2022 May 26;40(24):3305-3312. doi: 10.1016/j.vaccine.2022.04.065. Epub 2022 Apr 27. Vaccine. 2022. PMID: 35527057 Free PMC article.
-
Adverse Events Following COVID-19 Vaccination in Adolescents: Insights From Pharmacovigilance Study of VigiBase.J Korean Med Sci. 2024 Mar 4;39(8):e76. doi: 10.3346/jkms.2024.39.e76. J Korean Med Sci. 2024. PMID: 38442719 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Neurological Complications Following COVID-19 Vaccination.Curr Neurol Neurosci Rep. 2023 Jan;23(1):1-14. doi: 10.1007/s11910-022-01247-x. Epub 2022 Nov 29. Curr Neurol Neurosci Rep. 2023. PMID: 36445631 Free PMC article. Review.
-
SARS-CoV-2 pathophysiology and post-vaccination severity: a systematic review.Immunol Res. 2024 Dec 18;73(1):17. doi: 10.1007/s12026-024-09553-x. Immunol Res. 2024. PMID: 39692912
Cited by
-
Characteristics of Oral Adverse Effects following COVID-19 Vaccination and Similarities with Oral Symptoms in COVID-19 Patients: Taste and Saliva Secretory Disorders.Med Princ Pract. 2025;34(2):101-120. doi: 10.1159/000543182. Epub 2024 Dec 19. Med Princ Pract. 2025. PMID: 39701050 Free PMC article. Review.
-
Safety of COVID-19 Vaccines among People with History of Allergy: A European Active Surveillance Study.Vaccines (Basel). 2024 Sep 17;12(9):1059. doi: 10.3390/vaccines12091059. Vaccines (Basel). 2024. PMID: 39340089 Free PMC article.
-
An improvement of the safety profile of SARS-CoV-2 vaccines is desirable.Pharmacol Res Perspect. 2024 Oct;12(5):e70008. doi: 10.1002/prp2.70008. Pharmacol Res Perspect. 2024. PMID: 39263854 Free PMC article. No abstract available.
References
-
- COVID‐19 Vaccines: Safety Surveillance Manual. World Health Organization; 2020.
-
- WHO COVID‐19 dashboard. Accessed August 6, 2023. https://covid19.who.int/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

