Prolonged vs Intermittent Infusions of β-Lactam Antibiotics in Adults With Sepsis or Septic Shock: A Systematic Review and Meta-Analysis
- PMID: 38864162
- PMCID: PMC11170459
- DOI: 10.1001/jama.2024.9803
Prolonged vs Intermittent Infusions of β-Lactam Antibiotics in Adults With Sepsis or Septic Shock: A Systematic Review and Meta-Analysis
Abstract
Importance: There is uncertainty about whether prolonged infusions of β-lactam antibiotics improve clinically important outcomes in critically ill adults with sepsis or septic shock.
Objective: To determine whether prolonged β-lactam antibiotic infusions are associated with a reduced risk of death in critically ill adults with sepsis or septic shock compared with intermittent infusions.
Data sources: The primary search was conducted with MEDLINE (via PubMed), CINAHL, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov from inception to May 2, 2024.
Study selection: Randomized clinical trials comparing prolonged (continuous or extended) and intermittent infusions of β-lactam antibiotics in critically ill adults with sepsis or septic shock.
Data extraction and synthesis: Data extraction and risk of bias were assessed independently by 2 reviewers. Certainty of evidence was evaluated with the Grading of Recommendations Assessment, Development and Evaluation approach. A bayesian framework was used as the primary analysis approach and a frequentist framework as the secondary approach.
Main outcomes and measures: The primary outcome was all-cause 90-day mortality. Secondary outcomes included intensive care unit (ICU) mortality and clinical cure.
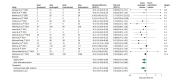
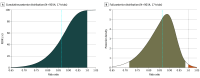
Results: From 18 eligible randomized clinical trials that included 9108 critically ill adults with sepsis or septic shock (median age, 54 years; IQR, 48-57; 5961 men [65%]), 17 trials (9014 participants) contributed data to the primary outcome. The pooled estimated risk ratio for all-cause 90-day mortality for prolonged infusions of β-lactam antibiotics compared with intermittent infusions was 0.86 (95% credible interval, 0.72-0.98; I2 = 21.5%; high certainty), with a 99.1% posterior probability that prolonged infusions were associated with lower 90-day mortality. Prolonged infusion of β-lactam antibiotics was associated with a reduced risk of intensive care unit mortality (risk ratio, 0.84; 95% credible interval, 0.70-0.97; high certainty) and an increase in clinical cure (risk ratio, 1.16; 95% credible interval, 1.07-1.31; moderate certainty).
Conclusions and relevance: Among adults in the intensive care unit who had sepsis or septic shock, the use of prolonged β-lactam antibiotic infusions was associated with a reduced risk of 90-day mortality compared with intermittent infusions. The current evidence presents a high degree of certainty for clinicians to consider prolonged infusions as a standard of care in the management of sepsis and septic shock.
Trial registration: PROSPERO Identifier: CRD42023399434.
Conflict of interest statement
Figures

Comment in
-
Resolving the Dilemma on Continuous vs Intermittent β-Lactam Antibiotics in Sepsis.JAMA. 2024 Aug 27;332(8):623-625. doi: 10.1001/jama.2024.10168. JAMA. 2024. PMID: 38864160 No abstract available.
References
-
- Roberts JA, Joynt GM, Lee A, et al. ; SMARRT Study Collaborators and the ANZICS Clinical Trials Group . The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: data from the Multinational Sampling Antibiotics in Renal Replacement Therapy study. Clin Infect Dis. 2021;72(8):1369-1378. doi: 10.1093/cid/ciaa224 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

