Sacituzumab govitecan vs. chemotherapy for metastatic breast cancer: a meta-analysis on safety outcomes
- PMID: 38864297
- PMCID: PMC11376414
- DOI: 10.1080/14796694.2024.2354162
Sacituzumab govitecan vs. chemotherapy for metastatic breast cancer: a meta-analysis on safety outcomes
Abstract
Aim: There is limited data available regarding the comparison of Sacituzumab govitecan (SG) vs. chemotherapy in metastatic breast cancer patients.Materials & methods: We performed a systematic review and meta-analysis aimed to assess the safety profile of SG vs. chemotherapy for metastatic breast cancer (mBC) clinical trials.Results: The pooled odds ratio for outcomes such as grade 3-4 and all grade neutropenia, leukopenia, anemia and other non-hematological adverse events showed a higher risk for patients receiving SG. No statistically significant differences were reported in terms of grade 3-4 fatigue, all grade nausea, febrile neutropenia and treatment discontinuation due to adverse events.Conclusion: Our data, coupled with a statistically and clinically meaningful survival benefit, support the use of SG for mBC.
Keywords: antibody–drug conjugate; breast; breast cancer; sacituzumab govitecan; triple-negative breast cancer.
Plain language summary
[Box: see text].
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
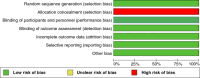
Figures




References
-
- Nicolò E, Repetto M, Boscolo Bielo L, Tarantino P, Curigliano G. Antibody–drug conjugates in breast cancer: what is beyond HER2? Cancer J. 2022;28(6):436–445. doi: 10.1097/PPO.0000000000000629 - DOI - PubMed
-
• A very interesting review discussing the role of antibody–drug conjugates in breast cancer patients.
-
- Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody–drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32(6):746–756. doi: 10.1016/j.annonc.2021.03.005 - DOI - PubMed
-
- Rugo HS, Tolaney SM, Loirat D, et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer. 2022;8(1):98. doi: 10.1038/s41523-022-00467-1 - DOI - PMC - PubMed
-
•• The practice-changing phase III ASCENT trial exploring sacituzumab govitecan for triple-negative patients.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
