Aptamer-Based DNA Allosteric Switch for Regulation of Protein Activity
- PMID: 38864341
- PMCID: PMC11321679
- DOI: 10.1002/advs.202402531
Aptamer-Based DNA Allosteric Switch for Regulation of Protein Activity
Abstract
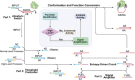
Allostery is a fundamental way to regulate the function of biomolecules playing crucial roles in cell metabolism and proliferation and is deemed the second secret of life. Given the limited understanding of the structure of natural allosteric molecules, the development of artificial allosteric molecules brings a huge opportunity to transform the allosteric mechanism into practical applications. In this study, the concept of bionics is introduced into the design of artificial allosteric molecules and an allosteric DNA switch with an activity site and an allosteric site based on two aptamers for selective inhibition of thrombin activity. Compared with the single aptamer, the allosteric switch possesses a significantly enhanced inhibition ability, which can be precisely regulated by converting the switch states. Moreover, the dynamic allosteric switch is further subjected to the control of the DNA threshold circuit for realizing automatic concentration determination and activity inhibition of thrombin. These compelling results confirm that this allosteric switch equipped with self-sensing and information-processing modules puts a new slant on the research of allosteric mechanisms and further application of allosteric tactics in chemical and biomedical fields.
Keywords: allostery; aptamer; bionics; enzyme activity regulation; molecular switch.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
- 22374136/National Natural Science Foundation of China
- 232300421021/Natural Science Foundation of Henan
- 242300421121/Natural Science Foundation of Henan
- 22TRTSTHN002/Program for Innovative Research Team (in Science and Technology) in University of Henan Province
- 2022YFC2009600/National Key Research and Development Program
LinkOut - more resources
Full Text Sources
