Bacterial histone HBb from Bdellovibrio bacteriovorus compacts DNA by bending
- PMID: 38864377
- PMCID: PMC11317129
- DOI: 10.1093/nar/gkae485
Bacterial histone HBb from Bdellovibrio bacteriovorus compacts DNA by bending
Abstract
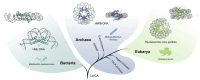
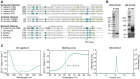
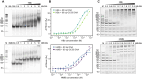
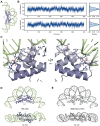
Histones are essential for genome compaction and transcription regulation in eukaryotes, where they assemble into octamers to form the nucleosome core. In contrast, archaeal histones assemble into dimers that form hypernucleosomes upon DNA binding. Although histone homologs have been identified in bacteria recently, their DNA-binding characteristics remain largely unexplored. Our study reveals that the bacterial histone HBb (Bd0055) is indispensable for the survival of Bdellovibrio bacteriovorus, suggesting critical roles in DNA organization and gene regulation. By determining crystal structures of free and DNA-bound HBb, we unveil its distinctive dimeric assembly, diverging from those of eukaryotic and archaeal histones, while also elucidating how it binds and bends DNA through interaction interfaces reminiscent of eukaryotic and archaeal histones. Building on this, by employing various biophysical and biochemical approaches, we further substantiated the ability of HBb to bind and compact DNA by bending in a sequence-independent manner. Finally, using DNA affinity purification and sequencing, we reveal that HBb binds along the entire genomic DNA of B. bacteriovorus without sequence specificity. These distinct DNA-binding properties of bacterial histones, showcasing remarkable similarities yet significant differences from their archaeal and eukaryotic counterparts, highlight the diverse roles histones play in DNA organization across all domains of life.
Plain language summary
Histones, traditionally known for organizing and regulating DNA in eukaryotes and archaea, have recently been discovered in bacteria, opening up a new frontier in our understanding of genome organization across the domains of life. Our study investigates the largely unexplored DNA-binding properties of bacterial histones, focusing on HBb in Bdellovibrio bacteriovorus. We reveal that HBb is essential for bacterial survival and exhibits DNA-binding properties similar to archaeal and eukaryotic histones. However, unlike eukaryotic and archaeal histones, which wrap DNA, HBb bends DNA without sequence specificity. This work not only broadens our understanding of DNA organization across different life forms but also suggests that bacterial histones may have diverse roles in genome organization.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
The chromatin landscape of the histone-possessing Bacteriovorax bacteria.Genome Res. 2025 Jan 22;35(1):109-123. doi: 10.1101/gr.279418.124. Genome Res. 2025. PMID: 39572228 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Melbournevirus encodes a shorter H2B-H2A doublet histone variant that forms structurally distinct nucleosome structures.Nat Commun. 2025 Jul 26;16(1):6903. doi: 10.1038/s41467-025-62031-2. Nat Commun. 2025. PMID: 40715086 Free PMC article.
-
Bacterial chromatin proteins, transcription, and DNA topology: Inseparable partners in the control of gene expression.Mol Microbiol. 2024 Jul;122(1):81-112. doi: 10.1111/mmi.15283. Epub 2024 Jun 7. Mol Microbiol. 2024. PMID: 38847475 Free PMC article. Review.
-
Identification, characterization and classification of prokaryotic nucleoid-associated proteins.Mol Microbiol. 2025 Mar;123(3):206-217. doi: 10.1111/mmi.15298. Epub 2024 Jul 22. Mol Microbiol. 2025. PMID: 39039769 Free PMC article. Review.
-
Histones and histone variant families in prokaryotes.Nat Commun. 2024 Sep 11;15(1):7950. doi: 10.1038/s41467-024-52337-y. Nat Commun. 2024. PMID: 39261503 Free PMC article.
-
CUT&Tag reveals unconventional G-quadruplex landscape in Mycobacterium tuberculosis in response to oxidative stress.Nat Commun. 2025 Aug 6;16(1):7253. doi: 10.1038/s41467-025-62485-4. Nat Commun. 2025. PMID: 40770179 Free PMC article.
References
-
- Luger K., Mader A.W., Richmond R.K., Sargent D.F., Richmond T.J.. Crystal structure of the nucleosome core particle at 2.8 angstrom resolution. Nature. 1997; 389:251–260. - PubMed
-
- Kornberg R.D., Thomas J.O.. Chromatin structure; oligomers of the histones. Science. 1974; 184:865–868. - PubMed
-
- Jenuwein T., Allis C.D.. Translating the histone code. Science. 2001; 293:1074–1080. - PubMed
-
- Luger K., Richmond T.J.. The histone tails of the nucleosome. Curr. Opin. Genet. Dev. 1998; 8:140–146. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

