Dexamethasone-tamoxifen combination exerts synergistic therapeutic effects in tamoxifen-resistance breast cancer cells
- PMID: 38864530
- PMCID: PMC11230869
- DOI: 10.1042/BSR20240367
Dexamethasone-tamoxifen combination exerts synergistic therapeutic effects in tamoxifen-resistance breast cancer cells
Abstract
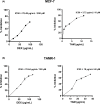
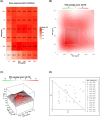
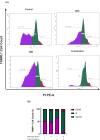
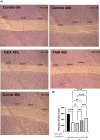
Tamoxifen (TAM) is a key player in estrogen receptor-positive (ER+) breast cancer (BC); however, ∼30% of patients experience relapse and a lower survival rate due to TAM resistance. TAM resistance was related to the over expression of SOX-2 gene, which is regulated by the E2F3 transcription factor in the Wnt signaling pathway. It was suggested that SOX-2 overexpression was suppressed by dexamethasone (DEX), a glucocorticoid commonly prescribed to BC patients. The aim of the present study is to explore the effect of combining DEX and TAM on the inhibition of TAM-resistant LCC-2 cells (TAMR-1) through modulating the E2F3/SOX-2-mediated Wnt signaling pathway. The effect of the combination therapy on MCF-7 and TAMR-1 cell viability was assessed. Drug interactions were analyzed using CompuSyn and SynergyFinder softwares. Cell cycle distribution, apoptotic protein expression, gene expression levels of SOX-2 and E2F3, and cell migration were also assessed. Combining DEX with TAM led to synergistic inhibition of TAMR-1 cell proliferation and migration, induced apoptosis, reduced SOX-2 and E2F3 expression and was also associated with S and G2-M phase arrest. Therefore, combining DEX with TAM may present an effective therapeutic option to overcome TAM resistance, by targeting the E2F3/SOX-2/Wnt signaling pathway, in addition to its anti-inflammatory effect.
Keywords: E2F3; SOX-2; breast cancer; dexamethasone; synergistic effect; tamoxifen-resistance.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

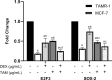
References
-
- (2022) Breast cancer n.d. https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on September 6, 2022)
-
- Ibrahim A.H. and Shash E. (2022) General oncology care in Egypt. Cancer Arab World 41–61 10.1007/978-981-16-7945-2_4 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

