Efficacy of rifapentine and other rifamycins against Coxiella burnetii in vitro
- PMID: 38864598
- PMCID: PMC11218529
- DOI: 10.1128/spectrum.01034-24
Efficacy of rifapentine and other rifamycins against Coxiella burnetii in vitro
Abstract
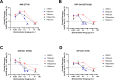
Since 1999, doxycycline and hydroxychloroquine have been the recommended treatment for chronic Q fever, a life-threatening disease caused by the bacterial pathogen, Coxiella burnetii. Despite the duration of its use, the treatment is not ideal due to the lengthy treatment time, high mortality rate, resistant strains, and the potential for contraindicated usage. A literature search was conducted to identify studies that screened large panels of drugs against C. burnetii to identify novel targets with potential efficacy against C. burnetii. Twelve candidate antimicrobials approved for use in humans by the US Food and Drug Administration were selected and minimum inhibitory concentrations (MICs) were determined against the low virulence strain Nine Mile phase II. Rifabutin and rifaximin were the best performing antibiotics tested with MICs of ≤0.01 µg mL-1. Further screening of these top candidates was conducted alongside two drugs from the same class, rifampin, well-characterized, and rifapentine, not previously reported against C. burnetii. These were screened against virulent strains of C. burnetii representing three clinically relevant genotypes. Rifapentine was the most effective in the human monocytic leukemia cell line, THP-1, with a MIC ≤0.01 µg mL-1. In the human kidney epithelial cell line, A-498, efficacy of rifapentine, rifampin, and rifabutin varied across C. burnetii strains with MICs between ≤0.001 and 0.01 µg mL-1. Rifampin, rifabutin, and rifapentine were all bactericidal against C. burnetii; however, rifabutin and rifapentine demonstrated impressive bactericidal activity as low as 0.1 µg mL-1 and should be further explored as alternative Q fever treatments given their efficacy in vitro.
Importance: This work will help inform investigators and physicians about potential alternative antimicrobial therapies targeting the causative agent of Q fever, Coxiella burnetii. Chronic Q fever is difficult to treat, and alternative antimicrobials are needed. This manuscript explores the efficacy of rifamycin antibiotics against virulent strains of C. burnetii representing three clinically relevant genotypes in vitro. Importantly, this study determines the susceptibility of C. burnetii to rifapentine, which has not been previously reported. Evaluation of the bactericidal activity of the rifamycins reveals that rifabutin and rifapentine are bactericidal at low concentrations, which is unusual for antibiotics against C. burnetii.
Keywords: Q fever; antibiotics; antimicrobial activity; bacterial infection; bactericidal activity; zoonotic infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Neurotransmitter System-Targeting Drugs Antagonize Growth of the Q Fever Agent, Coxiella burnetii, in Human Cells.mSphere. 2021 Aug 25;6(4):e0044221. doi: 10.1128/mSphere.00442-21. Epub 2021 Jul 7. mSphere. 2021. PMID: 34232075 Free PMC article.
-
Bacteriostatic and bactericidal activities of tigecycline against Coxiella burnetii and comparison with those of six other antibiotics.Antimicrob Agents Chemother. 2009 Jun;53(6):2690-2. doi: 10.1128/AAC.01424-08. Epub 2009 Mar 30. Antimicrob Agents Chemother. 2009. PMID: 19332671 Free PMC article.
-
In Vitro Activity of Rifampin, Rifabutin, and Rifapentine against Enterococci and Streptococci from Periprosthetic Joint Infection.Microbiol Spectr. 2021 Sep 3;9(1):e0007121. doi: 10.1128/Spectrum.00071-21. Epub 2021 Jul 14. Microbiol Spectr. 2021. PMID: 34259553 Free PMC article.
-
Clinical microbiology of Coxiella burnetii and relevant aspects for the diagnosis and control of the zoonotic disease Q fever.Vet Q. 2013;33(3):148-60. doi: 10.1080/01652176.2013.843809. Epub 2013 Oct 28. Vet Q. 2013. PMID: 24161079 Review.
-
[Drug activity against Coxiella burnetii, a pathogen of Q fever].Antibiot Khimioter. 2007;52(1-2):46-56. Antibiot Khimioter. 2007. PMID: 18461810 Review. Russian. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

