Investigating the importance of selected surface-exposed loops in HpuB for hemoglobin binding and utilization by Neisseria gonorrhoeae
- PMID: 38864605
- PMCID: PMC11238557
- DOI: 10.1128/iai.00211-24
Investigating the importance of selected surface-exposed loops in HpuB for hemoglobin binding and utilization by Neisseria gonorrhoeae
Abstract
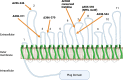
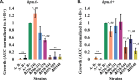
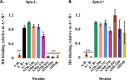
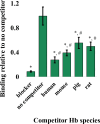
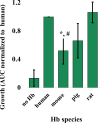
Neisseria gonorrhoeae is the etiological agent of the sexually transmitted infection gonorrhea. The pathogen is a global health challenge since no protective immunity results from infection, and far fewer treatment options are available with increasing antimicrobial resistance. With no efficacious vaccines, researchers are exploring new targets for vaccine development and innovative therapeutics. The outer membrane TonB-dependent transporters (TdTs) produced by N. gonorrhoeae are considered promising vaccine antigens as they are highly conserved and play crucial roles in overcoming nutritional immunity. One of these TdTs is part of the hemoglobin transport system comprised of HpuA and HpuB. This system allows N. gonorrhoeae to acquire iron from hemoglobin (hHb). In the current study, mutations in the hpuB gene were generated to better understand the structure-function relationships in HpuB. This study is one of the first to demonstrate that N. gonorrhoeae can bind to and utilize hemoglobin produced by animals other than humans. This study also determined that when HpuA is absent, mutations targeting extracellular loop 7 of HpuB led to defective hHb binding and utilization. However, when the lipoprotein HpuA is present, these loop 7 mutants recovered their ability to bind hHb, although the growth phenotype remained significantly impaired. Interestingly, loop 7 contains putative heme-binding motifs and a hypothetical α-helical region, both of which may be important for the use of hHb. Taken together, these results highlight the importance of loop 7 in the functionality of HpuB in binding hHb and extracting and internalizing iron.
Keywords: N. gonorrhoeae; N. meningitidis; TonB-dependent transporter; hemoglobin; hemoglobin–haptoglobin receptor; lipoprotein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Update of
-
Investigating the importance of surface exposed loops in the gonococcal HpuB transporter for hemoglobin binding and utilization.bioRxiv [Preprint]. 2023 Oct 30:2023.10.30.564842. doi: 10.1101/2023.10.30.564842. bioRxiv. 2023. Update in: Infect Immun. 2024 Jul 11;92(7):e0021124. doi: 10.1128/iai.00211-24. PMID: 37961140 Free PMC article. Updated. Preprint.
Similar articles
-
Investigating the importance of surface exposed loops in the gonococcal HpuB transporter for hemoglobin binding and utilization.bioRxiv [Preprint]. 2023 Oct 30:2023.10.30.564842. doi: 10.1101/2023.10.30.564842. bioRxiv. 2023. Update in: Infect Immun. 2024 Jul 11;92(7):e0021124. doi: 10.1128/iai.00211-24. PMID: 37961140 Free PMC article. Updated. Preprint.
-
Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation.mBio. 2024 May 8;15(5):e0011924. doi: 10.1128/mbio.00119-24. Epub 2024 Apr 9. mBio. 2024. PMID: 38587424 Free PMC article.
-
Point Mutations in TbpA Abrogate Human Transferrin Binding in Neisseria gonorrhoeae.Infect Immun. 2022 Nov 17;90(11):e0041422. doi: 10.1128/iai.00414-22. Epub 2022 Nov 2. Infect Immun. 2022. PMID: 36321833 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
Cited by
-
Exploring heme and iron acquisition strategies of Porphyromonas gingivalis-current facts and hypotheses.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf019. doi: 10.1093/femsre/fuaf019. FEMS Microbiol Rev. 2025. PMID: 40343779 Free PMC article. Review.
References
-
- WHO . 2021. Gonorrhoea: latest antimicrobial global surveillance results and guidance for vaccine development published. Available from: https://www.who.int/news/item/22-11-2021-gonorrhoea-antimicrobial-resist...
-
- CDC . 2023. National overview of STDs, 2021. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/std/statistics/2021/overview.htm#Gonorrhea
-
- CDC . 2021. Sexually transmitted infections prevalence, incidence, and cost estimates in the United States. Available from: https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

