Procyanidin B2 improves developmental capacity of bovine oocytes via promoting PPARγ/UCP1-mediated uncoupling lipid catabolism during in vitro maturation
- PMID: 38864666
- PMCID: PMC11533046
- DOI: 10.1111/cpr.13687
Procyanidin B2 improves developmental capacity of bovine oocytes via promoting PPARγ/UCP1-mediated uncoupling lipid catabolism during in vitro maturation
Abstract
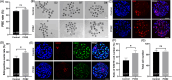
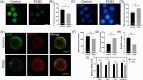
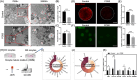
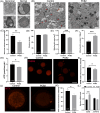
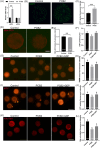
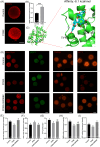
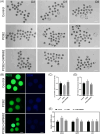
Metabolic balance is essential for oocyte maturation and acquisition of developmental capacity. Suboptimal conditions of in vitro cultures would lead to lipid accumulation and finally result in disrupted oocyte metabolism. However, the effect and mechanism underlying lipid catabolism in oocyte development remain elusive currently. In the present study, we observed enhanced developmental capacity in Procyanidin B2 (PCB2) treated oocytes during in vitro maturation. Meanwhile, reduced oxidative stress and declined apoptosis were found in oocytes after PCB2 treatment. Further studies confirmed that oocytes treated with PCB2 preferred to lipids catabolism, leading to a notable decrease in lipid accumulation. Subsequent analyses revealed that mitochondrial uncoupling was involved in lipid catabolism, and suppression of uncoupling protein 1 (UCP1) would abrogate the elevated lipid consumption mediated by PCB2. Notably, we identified peroxisome proliferator-activated receptor gamma (PPARγ) as a potential target of PCB2 by docking analysis. Subsequent mechanistic studies revealed that PCB2 improved oocyte development capacity and attenuated oxidative stress by activating PPARγ mediated mitochondrial uncoupling. Our findings identify that PCB2 intricately improves oocyte development capacity through targeted activation of the PPARγ/UCP1 pathway, fostering uncoupling lipid catabolism while concurrently mitigating oxidative stress.
© 2024 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Procyanidin B2 improves the in vitro maturation of porcine oocytes and subsequent embryonic development.Theriogenology. 2025 Oct 15;246:117555. doi: 10.1016/j.theriogenology.2025.117555. Epub 2025 Jun 25. Theriogenology. 2025. PMID: 40580853
-
Procyanidin B2 Activates PPARγ to Induce M2 Polarization in Mouse Macrophages.Front Immunol. 2019 Aug 7;10:1895. doi: 10.3389/fimmu.2019.01895. eCollection 2019. Front Immunol. 2019. PMID: 31440258 Free PMC article.
-
Procyanidin B1 promotes in vitro maturation of pig oocytes by reducing oxidative stress.Mol Reprod Dev. 2021 Jan;88(1):55-66. doi: 10.1002/mrd.23440. Epub 2020 Nov 25. Mol Reprod Dev. 2021. PMID: 33241626 Free PMC article.
-
The role of L-carnitine in the control of oxidative stress and lipid β-oxidation during in vitro follicle growth, oocyte maturation, embryonic development and cryopreservation: a review.Zygote. 2024 Oct;32(5):335-340. doi: 10.1017/S096719942400039X. Epub 2024 Nov 7. Zygote. 2024. PMID: 39506889 Review.
-
Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions.Int J Mol Sci. 2021 Mar 26;22(7):3421. doi: 10.3390/ijms22073421. Int J Mol Sci. 2021. PMID: 33810351 Free PMC article. Review.
References
-
- Del Collado M, Saraiva NZ, Lopes FL, et al. Influence of bovine serum albumin and fetal bovine serum supplementation during in vitro maturation on lipid and mitochondrial behaviour in oocytes and lipid accumulation in bovine embryos. Reprod Fertil Dev. 2015;28(11):1721‐1732. doi:10.1071/RD15067 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2021YFD1200402/National Key Research and Development Program of China
- 2022BC003/Key Technologies for Cryopreservation of Gametes and Embryos in Cattle and Sheep
- SKLSGIHP2021A01/Training and Guiding Outstanding Young and Middle-aged Talents
- nano-particles & 226Z7713G/Central Guidance on Local Science and Technology Development Fund
- H2023206029/Specific Project of Hebei Province for Outstanding Talents in Clinical Medicine, and Natural Science Foundation of Hebei province
LinkOut - more resources
Full Text Sources
Research Materials

