Genetic variants and down-regulation of CACNA1H in pheochromocytoma
- PMID: 38864697
- PMCID: PMC11301417
- DOI: 10.1530/ERC-23-0061
Genetic variants and down-regulation of CACNA1H in pheochromocytoma
Abstract
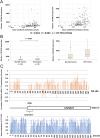
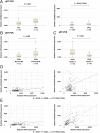
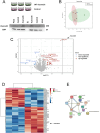
Pheochromocytoma (PCC) and abdominal paraganglioma (aPGL) (together abbreviated PPGL) frequently present with an underlying genetic event in a PPGL driver gene, and additional susceptibility genes are anticipated. Here, we re-analyzed whole-exome sequencing data for PCC patients and identified two patients with rare missense variants in the calcium voltage-gated channel subunit 1H gene (CACNA1H). CACNA1H variants were also found in the clinical setting in PCC patients using targeted sequencing and from analysis of The Cancer Genome Atlas database. In total, CACNA1H variants were found in six PCC cases. Three of these were constitutional, and two are known to have functional consequences on hormone production and gene expression in primary aldosteronism and aldosterone-producing adrenocortical adenoma. In general, PPGL exhibited reduced CACNA1H mRNA expression as compared to normal adrenal. Immunohistochemistry showed strong CACNA1H (CaV3.2) staining in adrenal medulla while PPGL typically had weak or negative staining. Reduced CACNA1H gene expression was especially pronounced in PCC compared to aPGL and in PPGL with cluster 2 kinase signaling phenotype. Furthermore, CACNA1H levels correlated with HIF1A and HIF2A. Moreover, TCGA data revealed a correlation between CACNA1H methylation density and gene expression. Expression of rCacna1h in PC12 cells induced differential protein expression profiles, determined by mass spectrometry, as well as a shift in the membrane potential where maximum calcium currents were observed, as determined by electrophysiology. The findings suggest the involvement of CACNA1H/CaV3.2 in pheochromocytoma development and establish a potential link between the etiology of adrenomedullary and adrenocortical tumor development.
Keywords: CACNA1H; calcium channel; paraganglioma; pheochromocytoma.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



References
-
- Clay MR, Pinto EM, Cline C, Tran QT, Lin T, Dyer MA, Shi L, Wu H, Pounds SB, Zambetti GP, et al.2019DNA methylation profiling reveals prognostically significant groups in pediatric adrenocortical tumors: a report from the international pediatric adrenocortical tumor registry. JCO Precision Oncology 3. (10.1200/PO.19.00163) - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

