Metastatic glioblastoma to the lungs: a case report and literature review
- PMID: 38864820
- PMCID: PMC11172249
- DOI: 10.1080/20450907.2024.2351789
Metastatic glioblastoma to the lungs: a case report and literature review
Abstract
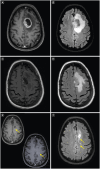
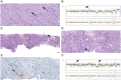
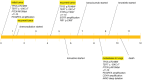
Glioblastoma is the most common malignant primary brain tumor. Despite its infiltrative nature, extra-cranial glioblastoma metastases are rare. We present a case of a 63-year-old woman with metastatic glioblastoma in the lungs. Sarcomatous histology, a reported risk factor for disseminated disease, was found. Genomic alterations of TP53 mutation, TERT mutation, PTEN mutation, and +7/-10 were also uncovered. Early evidence suggests these molecular aberrations are common in metastatic glioblastoma. Treatment with third-line lenvatinib resulted in a mixed response. This case contributes to the growing body of evidence for the role of genomic alterations in predictive risk in metastatic glioblastoma. There remains an unmet need for treatment of metastatic glioblastoma.
Keywords: VEGF; extra-neural glioblastoma; gliosarcoma; lenvatinib; metastatic glioblastoma; vascular endothelial growth factor inhibitor.
Plain language summary
Glioblastoma is the most common malignant primary brain tumor. Glioblastoma can spread into healthy tissue, but metastases beyond the brain are rare. We present a case of a 63-year-old woman with metastatic glioblastoma in the lungs. We identified risk factors associated with spread beyond the brain, including factors related to tissue structure and specific molecular alterations. Treatment with third-line lenvatinib resulted in a mixed response. This case adds to the limited existing data for the use of molecular alterations to serve as risk factors for metastatic glioblastoma. Treatment options are needed for this devastating disease.
Conflict of interest statement
Xiao-Tang Kong, MD received honorarium from Zai Lab for invited speeches for symposiums prior to July of 2021. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
