Successful Treatment of Severe Pemphigus Vulgaris with Reduced Side Effects Using a Novel IVIg Preparation
- PMID: 38865042
- PMCID: PMC11265024
- DOI: 10.1007/s13555-024-01191-3
Successful Treatment of Severe Pemphigus Vulgaris with Reduced Side Effects Using a Novel IVIg Preparation
Abstract
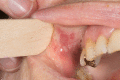
Pemphigus vulgaris (PV) is a rare autoimmune bullous dermatosis (AIBD) characterized by painful blistering of the skin and mucosa caused by autoantibodies that lead to loss of adhesion in the epidermis. Standard therapy for PV is corticosteroids, either alone or in combination with steroid-sparing immunosuppressants or infusions with rituximab. According to the published European guideline, high-dose intravenous immunoglobulin (IVIg) therapy with a dosage of 2 g per kg body weight distributed over 2-5 days every 4 weeks is a promising treatment option, especially for severe or refractory disease. This report describes a 73-year-old female patient with severe and recurrent disease who achieved stabilization with IVIg treatment. However, the patient experienced side effects such as headaches, nausea, and vomiting, which affected daily life. Hence, she was transitioned to a new IVIg preparation with a new manufacturing process, resulting in fewer side effects and an improved quality of life. Further follow-up is necessary to fully evaluate the effectiveness and tolerability of this new IVIg product.
Keywords: Autoimmune disease; High-dose intravenous immunoglobulins; IVIg; Pemphigus vulgaris; Side effects.
© 2024. The Author(s).
Conflict of interest statement
Alexander H. Enk received advisory board honoraria, consultancy fees and support for this publication from Biotest AG. Julia K. Winkler received travel expanse and honoraria from Biotest AG. Nadine Wiedenmayer and Anastasia S. Vollmer declare to have no competing interests.
Figures
Similar articles
-
Case report: A novel high-dose intravenous immunoglobulin preparation for the treatment of severe pemphigus vulgaris failing standard therapy.J Dermatol. 2024 Dec;51(12):1665-1668. doi: 10.1111/1346-8138.17475. Epub 2024 Sep 30. J Dermatol. 2024. PMID: 39349366 Free PMC article.
-
Efficacy of intravenous immunoglobulin (IVIG) affinity-purified anti-desmoglein anti-idiotypic antibodies in the treatment of an experimental model of pemphigus vulgaris.Clin Exp Immunol. 2010 Dec;162(3):543-9. doi: 10.1111/j.1365-2249.2010.04265.x. Epub 2010 Oct 21. Clin Exp Immunol. 2010. PMID: 20964642 Free PMC article.
-
Two Patients with Therapy-Resistant Pemphigus Vulgaris and Severe Underlying Disease Showing Good Response to a New IVIg Preparation.Dermatol Ther (Heidelb). 2025 Jan;15(1):237-244. doi: 10.1007/s13555-024-01326-6. Epub 2025 Jan 3. Dermatol Ther (Heidelb). 2025. PMID: 39751746 Free PMC article.
-
Current biologics in treatment of pemphigus foliaceus: a systematic review.Front Immunol. 2023 Oct 12;14:1267668. doi: 10.3389/fimmu.2023.1267668. eCollection 2023. Front Immunol. 2023. PMID: 37901249 Free PMC article.
-
High-Dose Intravenous Immunoglobulin in Skin Autoimmune Disease.Front Immunol. 2019 Jun 11;10:1090. doi: 10.3389/fimmu.2019.01090. eCollection 2019. Front Immunol. 2019. PMID: 31244821 Free PMC article. Review.
References
-
- van Beek N, Zillikens D, Schmidt E. Diagnosis of autoimmune bullous diseases. J Dtsch Dermatol Ges. 2018;16(9):1077–91. - PubMed
LinkOut - more resources
Full Text Sources