Time From Colorectal Cancer Surgery to Adjuvant Chemotherapy: Post Hoc Analysis of the SCOT Randomized Clinical Trial
- PMID: 38865139
- PMCID: PMC11170448
- DOI: 10.1001/jamasurg.2024.1555
Time From Colorectal Cancer Surgery to Adjuvant Chemotherapy: Post Hoc Analysis of the SCOT Randomized Clinical Trial
Abstract
Importance: The timing of adjuvant chemotherapy after surgery for colorectal cancer and its association with long-term outcomes have been investigated in national cohort studies, with no consensus on the optimal time from surgery to adjuvant chemotherapy.
Objective: To analyze the association between the timing of adjuvant chemotherapy after surgery for colorectal cancer and disease-free survival.
Design, setting, and participants: This is a post hoc analysis of the phase 3 SCOT randomized clinical trial, from 244 centers in 6 countries, investigating the noninferiority of 3 vs 6 months of adjuvant chemotherapy. Patients with high-risk stage II or stage III nonmetastatic colorectal cancer who underwent curative-intended surgery were randomized to either 3 or 6 months of adjuvant chemotherapy consisting of fluoropyrimidine and oxaliplatin regimens. Those with complete information on the date of surgery, treatment type, and long-term follow-up were investigated for the primary and secondary end points. Data were analyzed from May 2022 to February 2024.
Intervention: In the post hoc analysis, patients were grouped according to the start of adjuvant chemotherapy being less than 6 weeks vs greater than 6 weeks after surgery.
Main outcomes and measures: The primary end point was disease-free survival. The secondary end points were adverse events in the total treatment period or the first cycle of adjuvant chemotherapy.
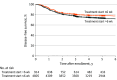
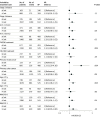
Results: A total of 5719 patients (2251 [39.4%] female; mean [SD] age, 63.4 [9.3] years) were included in the primary analysis after data curation; among them, 914 were in the early-start group and 4805 were in the late-start group. Median (IQR) follow-up was 72.0 (47.3-88.1) months, with a median (IQR) of 56 (41-66) days from surgery to chemotherapy. Five-year disease-free survival was 78.0% (95% CI, 75.3%-80.8%) in the early-start group and 73.2% (95% CI, 72.0%-74.5%) in the late-start group. In an adjusted Cox regression analysis, the start of adjuvant chemotherapy greater than 6 weeks after surgery was associated with worse disease-free survival (hazard ratio, 1.24; 95% CI, 1.06-1.46; P = .01). In adjusted logistic regression models, there was no association with adverse events in the total treatment period (odds ratio, 0.82; 95% CI, 0.65-1.04; P = .09) or adverse events in the first cycle of treatment (odds ratio, 0.77; 95% CI, 0.56-1.09; P = .13).
Conclusions and relevance: In this international population of patients with high-risk stage II and stage III colorectal cancer, starting adjuvant chemotherapy more than 6 weeks after surgery was associated with worse disease-free survival, with no difference in adverse events between the groups.
Trial registration: isrctn.org Identifier: ISRCTN59757862.
Conflict of interest statement
Figures
References
-
- Fitzmaurice C, Abate D, Abbasi N, et al. ; Global Burden of Disease Cancer Collaboration . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 2019;5(12):1749-1768. doi: 10.1001/jamaoncol.2019.2996 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

