Investigating Penetration and Antimicrobial Activity of Vector-Bicycle Conjugates
- PMID: 38865197
- PMCID: PMC11249977
- DOI: 10.1021/acsinfecdis.3c00427
Investigating Penetration and Antimicrobial Activity of Vector-Bicycle Conjugates
Abstract
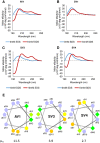
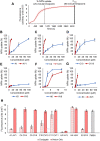
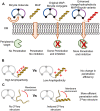
Growing antibiotic resistance is rapidly threatening the efficacy of treatments for Gram-negative infections. Bicycle molecules, constrained bicyclic peptides from diverse libraries generated by bacteriophage display that bind with high affinity to a chosen target are a potential new class of antibiotics. The generally impermeable bacterial outer membrane currently limits the access of peptides to bacteria. The conjugation of membrane active peptides offers an avenue for outer membrane penetration. Here, we investigate which physicochemical properties of a specific membrane active peptide (MAP), derived from ixosin-B, could be tweaked to enhance the penetration of conjugates by generating multiple MAP-Bicycle conjugate variants. We demonstrate that charge and hydrophobicity are important factors, which enhance penetration and, therefore, antimicrobial potency. Interestingly, we show that induction of secondary structure, but not a change in amphipathicity, is vital for effective penetration of the Gram-negative outer membrane. These results offer insights into the ways vectors could be designed to deliver Bicycle molecules (and other cargos) through biological membranes.
Keywords: antibiotics; antimicrobial peptides; antimicrobial resistance; bicycle; membrane active peptides; outer membrane.
Conflict of interest statement
The authors declare the following competing financial interest(s): H.N., N.L., C.R., N.B., S.J.S, M.J.S., and P.B. are shareholders and/or share option holders in Bicycle Therapeutics plc, the parent company of BicycleTx Ltd.
Figures
Similar articles
-
Plantaricin A, Derived from Lactiplantibacillus plantarum, Reduces the Intrinsic Resistance of Gram-Negative Bacteria to Hydrophobic Antibiotics.Appl Environ Microbiol. 2022 May 24;88(10):e0037122. doi: 10.1128/aem.00371-22. Epub 2022 May 2. Appl Environ Microbiol. 2022. PMID: 35499329 Free PMC article.
-
The Fellowship of the Rings: Macrocyclic Antibiotic Peptides Reveal an Anti-Gram-Negative Target.Biochemistry. 2020 Feb 4;59(4):343-345. doi: 10.1021/acs.biochem.9b01086. Epub 2020 Jan 7. Biochemistry. 2020. PMID: 31909599 Free PMC article.
-
Outer Membrane Disruption Overcomes Intrinsic, Acquired, and Spontaneous Antibiotic Resistance.mBio. 2020 Sep 22;11(5):e01615-20. doi: 10.1128/mBio.01615-20. mBio. 2020. PMID: 32963002 Free PMC article.
-
Breaching the Barrier: Quantifying Antibiotic Permeability across Gram-negative Bacterial Membranes.J Mol Biol. 2019 Aug 23;431(18):3531-3546. doi: 10.1016/j.jmb.2019.03.031. Epub 2019 Apr 5. J Mol Biol. 2019. PMID: 30959052 Review.
-
Antibiotics-Peptide Conjugates Against Multidrug-resistant Bacterial Pathogens.Curr Top Med Chem. 2018;18(22):1926-1936. doi: 10.2174/1568026619666181129141524. Curr Top Med Chem. 2018. PMID: 30499392 Review.
References
-
- Tacconelli E.; Carrara E.; Savoldi A.; Harbarth S.; Mendelson M.; Monnet D. L.; Pulcini C.; Kahlmeter G.; Kluytmans J.; Carmeli Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18 (3), 318–327. 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
-
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, 2015. - PubMed
-
- Wagstaff J. M.; Balmforth M.; Lewis N.; Dods R.; Rowland C.; Van Rietschoten K.; Chen L.; Harrison H.; Skynner M. J.; Dawson M.; et al. An Assay for Periplasm Entry Advances the Development of Chimeric Peptide Antibiotics. ACS Infect. Dis. 2020, 6 (9), 2355–2361. 10.1021/acsinfecdis.0c00389. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

