Replication of single viruses across the kingdoms, Fungi, Plantae, and Animalia
- PMID: 38865269
- PMCID: PMC11194502
- DOI: 10.1073/pnas.2318150121
Replication of single viruses across the kingdoms, Fungi, Plantae, and Animalia
Abstract
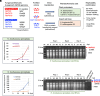
It is extremely rare that a single virus crosses host barriers across multiple kingdoms. Based on phylogenetic and paleovirological analyses, it has previously been hypothesized that single members of the family Partitiviridae could cross multiple kingdoms. Partitiviridae accommodates members characterized by their simple bisegmented double-stranded RNA genome; asymptomatic infections of host organisms; the absence of an extracellular route for entry in nature; and collectively broad host range. Herein, we show the replicability of single fungal partitiviruses in three kingdoms of host organisms: Fungi, Plantae, and Animalia. Betapartitiviruses of the phytopathogenic fungusRosellinia necatrix could replicate in protoplasts of the carrot (Daucus carota), Nicotiana benthamiana and Nicotiana tabacum, in some cases reaching a level detectable by agarose gel electrophoresis. Moreover, betapartitiviruses showed more robust replication than the tested alphapartitiviruses. One of the fungal betapartitiviruses, RnPV18, could persistently and stably infect carrot plants regenerated from virion-transfected protoplasts. Both alpha- and betapartitiviruses, although with different host preference, could replicate in two insect cell lines derived from the fall armyworm Spodoptera frugiperda and the fruit fly Drosophila melanogaster. Our results indicate the replicability of single partitiviruses in members of three kingdoms and provide insights into virus adaptation, host jumping, and evolution.
Keywords: Animalia; Plantae; cross-kingdom infection; fungal virus; partitivirus.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


Similar articles
-
A circular single-stranded DNA mycovirus infects plants and confers broad-spectrum fungal resistance.Mol Plant. 2024 Jun 3;17(6):955-971. doi: 10.1016/j.molp.2024.05.003. Epub 2024 May 13. Mol Plant. 2024. PMID: 38745413
-
Omnipresence of Partitiviruses in Rice Aggregate Sheath Spot Symptom-Associated Fungal Isolates from Paddies in Thailand.Viruses. 2021 Nov 12;13(11):2269. doi: 10.3390/v13112269. Viruses. 2021. PMID: 34835075 Free PMC article.
-
Distribution of Viruses Inhabiting Heterobasidion annosum in a Pine-Dominated Forest Plot in Southern Finland.Microb Ecol. 2018 Apr;75(3):622-630. doi: 10.1007/s00248-017-1027-6. Epub 2017 Aug 5. Microb Ecol. 2018. PMID: 28779297
-
Taxonomic reorganization of family Partitiviridae and other recent progress in partitivirus research.Virus Res. 2014 Aug 8;188:128-41. doi: 10.1016/j.virusres.2014.04.007. Epub 2014 Apr 21. Virus Res. 2014. PMID: 24768846 Review.
-
Evolutionary and ecological links between plant and fungal viruses.New Phytol. 2019 Jan;221(1):86-92. doi: 10.1111/nph.15364. Epub 2018 Aug 7. New Phytol. 2019. PMID: 30084143 Review.
Cited by
-
Characterization of a multi-segmented rod-shaped mycovirus within the order Martellivirales largely accommodating plant viruses.Virus Res. 2025 Jul;357:199591. doi: 10.1016/j.virusres.2025.199591. Epub 2025 May 30. Virus Res. 2025. PMID: 40451551 Free PMC article.
-
Characterization of an unusual tobacco rattle virus isolate and a novel phenuivirid in the Jerusalem sage.Virol J. 2025 Aug 6;22(1):269. doi: 10.1186/s12985-025-02896-3. Virol J. 2025. PMID: 40770347 Free PMC article.
-
Mycologists and Virologists Align: Proposing Botrytis cinerea for Global Mycovirus Studies.Viruses. 2024 Sep 18;16(9):1483. doi: 10.3390/v16091483. Viruses. 2024. PMID: 39339959 Free PMC article. Review.
-
A Hypovirulence-Associated Partitivirus and Re-Examination of Horizontal Gene Transfer Between Partitiviruses and Cellular Organisms.Int J Mol Sci. 2025 Apr 18;26(8):3853. doi: 10.3390/ijms26083853. Int J Mol Sci. 2025. PMID: 40332509 Free PMC article.
-
The virome of the panglobal, wide host-range plant pathogen Phytophthora cinnamomi: phylogeography and evolutionary insights.Virus Evol. 2025 Apr 1;11(1):veaf020. doi: 10.1093/ve/veaf020. eCollection 2025. Virus Evol. 2025. PMID: 40352162 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

