Randomized trial to compare acceptability of magnesium sulphate administration for preeclampsia and eclampsia: Springfusor pump versus standard of care
- PMID: 38865319
- PMCID: PMC11168672
- DOI: 10.1371/journal.pone.0286361
Randomized trial to compare acceptability of magnesium sulphate administration for preeclampsia and eclampsia: Springfusor pump versus standard of care
Abstract
Introduction: In low-resource settings, magnesium sulphate (MgSO4) for preeclampsia is administered majorly through an injection into the gluteal muscles 4-hourly for 24 hours. The repeated injections are very painful and may lead to infection, abscess formation, and reduced compliance.
Objective: To determine the acceptability of Springfusor® pump for the administration of Magnesium Sulphate in preeclampsia and eclampsia.
Design: Randomized Open Label Clinical Trial.
Methods: The study was conducted at Kawempe National Referral Hospital. Eligible women had a systolic blood pressure of ≥140mmHg and or diastolic blood pressure >90mmHg, proteinuria ≥+1, and the physician's decision to start on MgSO4. Four-hundred-ninety-six participants were randomized to a Springfusor® pump group (n = 248) or control (standard of care) (n = 248) administration of MgSO4. Intervention group had a loading dose (4gm of 50% MgSO4 intravenously over 20 minutes) and maintenance therapy (1gm of 50% MgSO4 intravenously per hour for 24 hours) administered using the Springfusor®. The standard of care (SOC) group received a loading dose of 4gm of 20% MgSO4 IV over 15-20 minutes, followed by 10gm of 50% MgSO4 intramuscular (5gm in each buttock) and a maintenance dose of 5gm of 50% MgSO4 was administered IM every 4 hours for 24 hours. Both arms received the rest of the care for preeclampsia/eclampsia as per the hospital guidelines. Acceptability of the method of administration was assessed using a Likert scale (1-5; 1 and 2: acceptable and 3-5: unacceptable). Pain at the site of MgSO4 administration was assessed using a Visual Analogue Scale 1-7, (1 minimal pain and 7 worst pain). Comparisons were assessed with the Chi-square test, Mann Whitney-Wilcoxon test, and Students' t-test.
Results: Intervention arm; was more acceptable than the standard of care arm, (95.3% vs70.3%; p<0.001), had a lower median pain score, (2(CI: 2-2), vs 4(CI: 4-5) p<0.001), and fewer side effects. Maternal mortality was comparable between groups (0.8% in the intervention arm vs 1.2% in the IM arm).
Trial registration: Trial No PACTR201712002887266 (https://pactr.samrc.ac.za/).
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
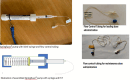

Similar articles
-
Administration patterns of magnesium sulphate for women with preeclampsia and immediate newborn outcomes in Kawempe National Referral Hospital-Uganda: a cohort study.BMC Pregnancy Childbirth. 2024 Nov 14;24(1):753. doi: 10.1186/s12884-024-06915-z. BMC Pregnancy Childbirth. 2024. PMID: 39543563 Free PMC article.
-
A novel 12-hour versus 24-hour magnesium sulfate regimen in the management of eclampsia and preeclampsia in Ghana (MOPEP Study): A randomized controlled trial.Int J Gynaecol Obstet. 2022 Nov;159(2):495-504. doi: 10.1002/ijgo.14181. Epub 2022 Apr 6. Int J Gynaecol Obstet. 2022. PMID: 35304745 Clinical Trial.
-
Treatment approaches for preeclampsia in low-resource settings: A randomized trial of the Springfusor pump for delivery of magnesium sulfate.Pregnancy Hypertens. 2012 Jan;2(1):32-8. doi: 10.1016/j.preghy.2011.09.002. Epub 2011 Oct 11. Pregnancy Hypertens. 2012. PMID: 26104987
-
Magnesium sulfate in eclampsia and pre-eclampsia: pharmacokinetic principles.Clin Pharmacokinet. 2000 Apr;38(4):305-14. doi: 10.2165/00003088-200038040-00002. Clin Pharmacokinet. 2000. PMID: 10803454 Review.
-
Clinical pharmacokinetic properties of magnesium sulphate in women with pre-eclampsia and eclampsia.BJOG. 2016 Feb;123(3):356-66. doi: 10.1111/1471-0528.13753. Epub 2015 Nov 24. BJOG. 2016. PMID: 26599617 Free PMC article. Review.
References
-
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American journal of obstetrics and gynecology. 2000;183(1):S1–S22. . - PubMed
-
- Mousa A, Mandili RL, Aljahdali M, Gari S, Khaimi S, Alahdal S, et al.. Maternal and Fetal Outcomes of Preeclampsia With and Without Severe Features in King Abdulaziz University Hospital, Jeddah, Saudi Arabia: A Retrospective Study. Cureus. 2022;14(11):e31013. Epub 2022/12/08. doi: 10.7759/cureus.31013 ; PubMed Central PMCID: PMC9717715. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

