Non-coding RNA yREX3 from human extracellular vesicles exerts macrophage-mediated cardioprotection via a novel gene-methylating mechanism
- PMID: 38865332
- PMCID: PMC11297535
- DOI: 10.1093/eurheartj/ehae357
Non-coding RNA yREX3 from human extracellular vesicles exerts macrophage-mediated cardioprotection via a novel gene-methylating mechanism
Abstract
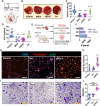
Background and aims: Extracellular vesicles (EVs) secreted by cardiosphere-derived cells exert immunomodulatory effects through the transmission of small non-coding RNAs.
Methods: The mechanism and role of yREX3, a small Y RNA abundant in EVs in myocardial injury, was investigated.
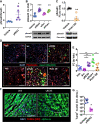
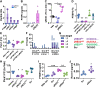
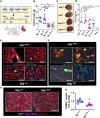
Results: yREX3 attenuates cardiac ischaemic injury by selective DNA methylation. Synthetic yREX3 encapsulated in lipid nanoparticles triggers broad transcriptomic changes in macrophages, localizes to the nucleus, and mediates epigenetic silencing of protein interacting with C kinase-1 (Pick1) through methylation of upstream CpG sites. Moreover, yREX3 interacts with polypyrimidine tract binding protein 3 (PTBP3) to methylate the Pick1 gene locus in a DNA methyltransferase-dependent manner. Suppression of Pick1 in macrophages potentiates Smad3 signalling and enhances efferocytosis, minimizing heart necrosis in rats with myocardial infarction. Adoptive transfer of Pick1-deficient macrophages recapitulates the cardioprotective effects of yREX3 in vivo.
Conclusions: These findings highlight the role of a small Y RNA mined from EVs with a novel gene-methylating mechanism.
Keywords: Efferocytosis; Inflammation; Macrophages; Myocardial infarction; Pick1; Small non-coding RNA.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures