Sex-dependent regulation of vertebrate somatic growth and aging by germ cells
- PMID: 38865462
- PMCID: PMC11168456
- DOI: 10.1126/sciadv.adi1621
Sex-dependent regulation of vertebrate somatic growth and aging by germ cells
Abstract
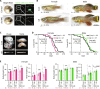
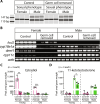
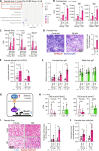
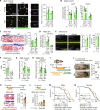
The function of germ cells in somatic growth and aging has been demonstrated in invertebrate models but remains unclear in vertebrates. We demonstrated sex-dependent somatic regulation by germ cells in the short-lived vertebrate model Nothobranchius furzeri. In females, germ cell removal shortened life span, decreased estrogen, and increased insulin-like growth factor 1 (IGF-1) signaling. In contrast, germ cell removal in males improved their health with increased vitamin D signaling. Body size increased in both sexes but was caused by different signaling pathways, i.e., IGF-1 and vitamin D in females and males, respectively. Thus, vertebrate germ cells regulate somatic growth and aging through different pathways of the endocrine system, depending on the sex, which may underlie the sexual difference in reproductive strategies.
Figures
References
-
- Williams G. C., Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690 (1996).
-
- Mukhopadhyay A., Tissenbaum H. A., Reproduction and longevity: Secrets revealed by C. elegans. Trends Cell Biol. 17, 65–71 (2007). - PubMed
-
- Kirkwood T. B., Evolution of ageing. Nature 270, 301–304 (1977). - PubMed
-
- Kirkwood T. B., Holliday R., The evolution of ageing and longevity. Proc. R. Soc. Lond. B Biol. Sci. 205, 531–546 (1979). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

