Formulation and assessment of biological properties of garcinia indica fruit extract mouthrinse as an adjunct to oral hygiene regimen: an in vitro analysis
- PMID: 38865512
- PMCID: PMC11189596
- DOI: 10.1590/1678-7757-2023-0291
Formulation and assessment of biological properties of garcinia indica fruit extract mouthrinse as an adjunct to oral hygiene regimen: an in vitro analysis
Abstract
The prevalence of gingivitis is substantial within the general population, necessitating rigorous oral hygiene maintenance.
Objective: This study assessed a Garcinia indica (GI) fruit extract-based mouthrinse, comparing it to a 0.1% turmeric mouthrinse and a 0.2% Chlorhexidine (CHX) mouthrinse. The evaluation encompassed substantivity, staining potential, antimicrobial efficacy and cytocompatibility.
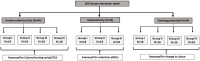
Methodology: The study employed 182 tooth sections. For antimicrobial analysis, 64 extracted human teeth coated with a polymicrobial biofilm were divided into four groups, each receiving an experimental mouthrinse or serving as a control group with distilled water. Microbial reduction was assessed through colony forming units (CFU). Substantivity was evaluated on 54 human tooth sections using a UV spectrophotometer, while staining potential was examined on 64 tooth sections. Cytocompatibility was tested using colorimetric assay to determine non-toxic levels of 0.2% GI fruit extract, 0.1% Turmeric, and 0.2% CHX.
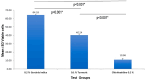
Results: Data were analysed with one-way ANOVA (α=0.05). Cell viability was highly significant (p<0.001) in the 0.2% GI group (64.1±0.29) compared to 0.1% Turmeric (40.2±0.34) and 0.2% CHX (10.95±1.40). For antimicrobial activity, both 0.2% GI (20.18±4.81) and 0.2% CHX (28.22±5.41) exhibited no significant difference (P>0.05) at end of 12 hours. However, 0.1% Turmeric showed minimal CFU reduction (P<0.001). Substantivity results at 360 minutes indicated statistically significant higher mean release rate in 0.1%Turmeric (12.47±5.84 ) when compared to 0.2% GI (5.02±3.04) and 0.2% CHX (4.13±2.25) (p<0.001). The overall discoloration changes (∆E) were more prominent in the 0.2% CHX group (18.65±8.3) compared to 0.2% GI (7.61±2.4) and 0.1% Turmeric (7.32±4.9) (P<0.001).
Conclusion: This study supports 0.2% GI and 0.1% Turmeric mouth rinses as potential natural alternatives to chemical mouth rinses. These findings highlight viability of these natural supplements in oral healthcare.
Conflict of interest statement
Conflicts of interest
The authors declare no conflicts of interest.
Figures
Similar articles
-
Evaluation of a mouthrinse containing guava leaf extract as part of comprehensive oral care regimen- a randomized placebo-controlled clinical trial.BMC Complement Altern Med. 2019 Nov 21;19(1):327. doi: 10.1186/s12906-019-2745-8. BMC Complement Altern Med. 2019. PMID: 31752836 Free PMC article. Clinical Trial.
-
Chlorhexidine substantivity on salivary flora and plaque-like biofilm: an in situ model.PLoS One. 2013 Dec 27;8(12):e83522. doi: 10.1371/journal.pone.0083522. eCollection 2013. PLoS One. 2013. PMID: 24386220 Free PMC article.
-
In vitro effect of chlorhexidine mouth rinses on polyspecies biofilms.Schweiz Monatsschr Zahnmed. 2011;121(5):432-41. Schweiz Monatsschr Zahnmed. 2011. PMID: 21656386 English, German.
-
The antiplaque/anticariogenic efficacy of Salvadora persica (Miswak) mouthrinse in comparison to that of chlorhexidine: a systematic review and meta-analysis.BMC Oral Health. 2019 Apr 27;19(1):64. doi: 10.1186/s12903-019-0741-5. BMC Oral Health. 2019. PMID: 31029127 Free PMC article.
-
Efficacy of chlorhexidine rinses after periodontal or implant surgery: a systematic review.Clin Oral Investig. 2019 Jan;23(1):21-32. doi: 10.1007/s00784-018-2761-y. Epub 2018 Dec 7. Clin Oral Investig. 2019. PMID: 30535817
References
-
- Osso D, Kanani N. Antiseptic mouth rinses: an update on comparative effectiveness, risks and recommendations. J Dent Hyg . 2013;87(1):10–18. - PubMed
-
- Langa GP, Muniz FW, Costa RD, Silveira TM, Rösing CK. The effect of cetylpyridinium chloride mouthrinse as adjunct to toothbrushing compared to placebo on interproximal plaque and gingival inflammation - a systematic review with meta-analyses. Clinical Oral Investig . 2021;25:745–757. doi: 10.1007/s00784-020-03661-2. - DOI - PubMed
-
- Haydari M, Bardakci AG, Koldsland OC, Aass AM, Sandvik L, Preus HR. Comparing the effect of 0.06% -, 0.12% and 0.2% chlorhexidine on plaque, bleeding and side effects in an experimental gingivitis model: a parallel group, double masked randomized clinical trial. 118 BMC Oral Health . 2017;17(1) doi: 10.1186/s12903-017-0400-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical