Less is better: various means to reduce protein load in the endoplasmic reticulum
- PMID: 38865586
- PMCID: PMC11880973
- DOI: 10.1111/febs.17201
Less is better: various means to reduce protein load in the endoplasmic reticulum
Abstract
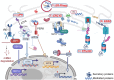
The endoplasmic reticulum (ER) is an important organelle that controls the intracellular and extracellular environments. The ER is responsible for folding almost one-third of the total protein population in the eukaryotic cell. Disruption of ER-protein folding is associated with numerous human diseases, including metabolic disorders, neurodegenerative diseases, and cancer. During ER perturbations, the cells deploy various mechanisms to increase the ER-folding capacity and reduce ER-protein load by minimizing the number of substrates entering the ER to regain homeostasis. These mechanisms include signaling pathways, degradation mechanisms, and other processes that mediate the reflux of ER content to the cytosol. In this review, we will discuss the recent discoveries of five different ER quality control mechanisms, including the unfolded protein response (UPR), ER-associated-degradation (ERAD), pre-emptive quality control, ER-phagy and ER to cytosol signaling (ERCYS). We will discuss the roles of these processes in decreasing ER-protein load and inter-mechanism crosstalk.
Keywords: COPII; ERAD; ERCYS; ER‐Phagy; ER‐pQC; UPR.
© 2024 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rapoport TA (2007) Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450, 663–669. - PubMed
-
- Deshaies RJ, Sanders SL, Feldheim DA & Schekman R (1991) Assembly of yeast sec proteins involved in translocation into the endoplasmic reticulum into a membrane‐bound multisubunit complex. Nature 349, 806–808. - PubMed
-
- Wickner W (1979) The assembly of proteins into biological membranes: the membrane trigger hypothesis. Annu Rev Biochem 48, 23–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

