Sex-specific biphasic alpha-synuclein response and alterations of interneurons in a COVID-19 hamster model
- PMID: 38865747
- PMCID: PMC11293593
- DOI: 10.1016/j.ebiom.2024.105191
Sex-specific biphasic alpha-synuclein response and alterations of interneurons in a COVID-19 hamster model
Abstract
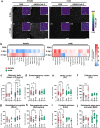
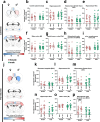
Background: Coronavirus disease 2019 (COVID-19) frequently leads to neurological complications after recovery from acute infection, with higher prevalence in women. However, mechanisms by which SARS-CoV-2 disrupts brain function remain unclear and treatment strategies are lacking. We previously demonstrated neuroinflammation in the olfactory bulb of intranasally infected hamsters, followed by alpha-synuclein and tau accumulation in cortex, thus mirroring pathogenesis of neurodegenerative diseases such as Parkinson's or Alzheimer's disease.
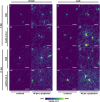
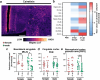
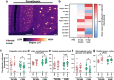
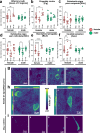
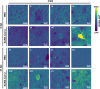
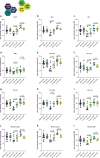
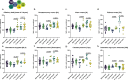
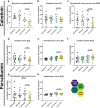
Methods: To uncover the sex-specific spatiotemporal profiles of neuroinflammation and neuronal dysfunction following intranasal SARS-CoV-2 infection, we quantified microglia cell density, alpha-synuclein immunoreactivity and inhibitory interneurons in cortical regions, limbic system and basal ganglia at acute and late post-recovery time points.
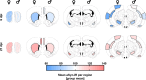
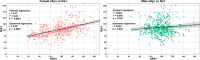
Findings: Unexpectedly, microglia cell density and alpha-synuclein immunoreactivity decreased at 6 days post-infection, then rebounded to overt accumulation at 21 days post-infection. This biphasic response was most pronounced in amygdala and striatum, regions affected early in Parkinson's disease. Several brain regions showed altered densities of parvalbumin and calretinin interneurons which are involved in cognition and motor control. Of note, females appeared more affected.
Interpretation: Our results demonstrate that SARS-CoV-2 profoundly disrupts brain homeostasis without neuroinvasion, via neuroinflammatory and protein regulation mechanisms that persist beyond viral clearance. The regional patterns and sex differences are in line with neurological deficits observed after SARS-CoV-2 infection.
Funding: Federal Ministry of Health, Germany (BMG; ZMV I 1-2520COR501 to G.G.), Federal Ministry of Education and Research, Germany (BMBF; 03COV06B to G.G.), Ministry of Science and Culture of Lower Saxony in Germany (14-76403-184, to G.G. and F.R.).
Keywords: Neurodegenerative disease; Neuroinflammation; Neurological symptoms; Post COVID-19 condition.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model.EBioMedicine. 2022 May;79:103999. doi: 10.1016/j.ebiom.2022.103999. Epub 2022 Apr 16. EBioMedicine. 2022. PMID: 35439679 Free PMC article.
-
Intravenous SARS-CoV-2 Spike protein induces neuroinflammation and alpha-Synuclein accumulation in brain regions relevant to Parkinson's disease.Brain Behav Immun. 2025 May 20;129:102-123. doi: 10.1016/j.bbi.2025.05.021. Online ahead of print. Brain Behav Immun. 2025. PMID: 40404020
-
Investigating post-infection anxiety- and depression-like behaviors in a SARS-CoV-2 mouse model.Theranostics. 2025 Apr 21;15(12):5738-5755. doi: 10.7150/thno.102752. eCollection 2025. Theranostics. 2025. PMID: 40365287 Free PMC article.
-
Exploring the Paradox of COVID-19 in Neurological Complications with Emphasis on Parkinson's and Alzheimer's Disease.Oxid Med Cell Longev. 2022 Aug 31;2022:3012778. doi: 10.1155/2022/3012778. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36092161 Free PMC article. Review.
-
The effect of SARS-CoV-2 on the development of Parkinson's disease: the role of α-synuclein.Hum Cell. 2024 Jan;37(1):1-8. doi: 10.1007/s13577-023-00988-2. Epub 2023 Sep 21. Hum Cell. 2024. PMID: 37735344 Review.
Cited by
-
SARS-CoV-2 Infection and Alpha-Synucleinopathies: Potential Links and Underlying Mechanisms.Int J Mol Sci. 2024 Nov 10;25(22):12079. doi: 10.3390/ijms252212079. Int J Mol Sci. 2024. PMID: 39596147 Free PMC article. Review.
-
Anti-Inflammatory and Neuroprotective Polyphenols Derived from the European Olive Tree, Olea europaea L., in Long COVID and Other Conditions Involving Cognitive Impairment.Int J Mol Sci. 2024 Oct 14;25(20):11040. doi: 10.3390/ijms252011040. Int J Mol Sci. 2024. PMID: 39456822 Free PMC article. Review.
-
Animal Models of Non-Respiratory, Post-Acute Sequelae of COVID-19.Viruses. 2025 Jan 14;17(1):98. doi: 10.3390/v17010098. Viruses. 2025. PMID: 39861887 Free PMC article. Review.
-
Infections in the Etiology of Parkinson's Disease and Synucleinopathies: A Renewed Perspective, Mechanistic Insights, and Therapeutic Implications.J Parkinsons Dis. 2024;14(7):1301-1329. doi: 10.3233/JPD-240195. J Parkinsons Dis. 2024. PMID: 39331109 Free PMC article. Review.
-
Neuroimmune pathophysiology of long COVID.Psychiatry Clin Neurosci. 2025 Jun 19:10.1111/pcn.13855. doi: 10.1111/pcn.13855. Online ahead of print. Psychiatry Clin Neurosci. 2025. PMID: 40536011 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

