Development of nitroalkene-based inhibitors to target STING-dependent inflammation
- PMID: 38865901
- PMCID: PMC11215336
- DOI: 10.1016/j.redox.2024.103202
Development of nitroalkene-based inhibitors to target STING-dependent inflammation
Abstract
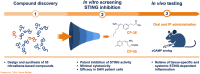
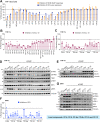
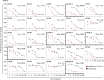
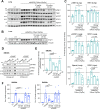
Stimulator of Interferon Genes (STING) is essential for the inflammatory response to cytosolic DNA. Despite that aberrant activation of STING is linked to an increasing number of inflammatory diseases, the development of inhibitors has been challenging, with no compounds in the pipeline beyond the preclinical stage. We previously identified endogenous nitrated fatty acids as novel reversible STING inhibitors. With the aim of improving the specificity and efficacy of these compounds, we developed and tested a library of nitroalkene-based compounds for in vitro and in vivo STING inhibition. The structure-activity relationship study revealed a robustly improved electrophilicity and reduced degrees of freedom of nitroalkenes by conjugation with an aromatic moiety. The lead compounds CP-36 and CP-45, featuring a β-nitrostyrene moiety, potently inhibited STING activity in vitro and relieved STING-dependent inflammation in vivo. This validates the potential for nitroalkene compounds as drug candidates for STING modulation to treat STING-driven inflammatory diseases, providing new robust leads for preclinical development.
Keywords: Drug discovery; Interferon; Nitroalkene-based compounds; STING inhibitors; STING-associated vasculopathy with onset in infancy (SAVI); Stimulator of Interferon Genes (STING).
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Francisco J Schopfer reports a relationship with Creegh Pharmaceuticals Inc that includes: board membership and equity or stocks. Francisco J Schopfer & Fei Chang reports a relationship with Furanica Inc that includes: board membership and equity or stocks. Christian K Holm reports a relationship with UV Medico that includes: consulting or advisory and employment. Francisco J Schopfer, Fei Chang, Christian K Holm, Anne Louise Hansen, Sonia R Salvatore, Luis Villacorta are the inventors of a patent application related to the subject matter of this manuscript. The University of Pittsburgh is the lead institution for this patent application, with joint ownership also held by Aarhus University and Morehouse School of Medicine. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

