Activation of Sirtuin3 by honokiol ameliorates alveolar epithelial cell senescence in experimental silicosis via the cGAS-STING pathway
- PMID: 38865904
- PMCID: PMC11215422
- DOI: 10.1016/j.redox.2024.103224
Activation of Sirtuin3 by honokiol ameliorates alveolar epithelial cell senescence in experimental silicosis via the cGAS-STING pathway
Abstract
Background: Silicosis, characterized by interstitial lung inflammation and fibrosis, poses a significant health threat. ATII cells play a crucial role in alveolar epithelial repair and structural integrity maintenance. Inhibiting ATII cell senescence has shown promise in silicosis treatment. However, the mechanism behind silica-induced senescence remains elusive.
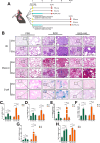
Methods: The study employed male C57BL/6 N mice and A549 human alveolar epithelial cells to investigate silicosis and its potential treatment. Silicosis was induced in mice via intratracheal instillation of crystalline silica particles, with honokiol administered intraperitoneally for 14 days. Silica-induced senescence in A549 cells was confirmed, and SIRT3 knockout and overexpression cell lines were generated. Various analyses were conducted, including immunoblotting, qRT-PCR, histology, and transmission electron microscopy. Statistical significance was determined using one-way ANOVA with Tukey's post-hoc test.
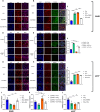
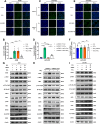
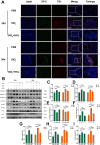
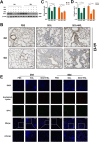
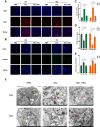
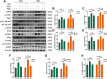
Results: This study elucidates how silica induces ATII cell senescence, emphasizing mtDNA damage. Notably, honokiol (HKL) emerges as a promising anti-senescence and anti-fibrosis agent, acting through sirt3. honokiol effectively attenuated senescence in ATII cells, dependent on sirt3 expression, while mitigating mtDNA damage. Sirt3, a class III histone deacetylase, regulates senescence and mitochondrial stress. HKL activates sirt3, protecting against pulmonary fibrosis and mitochondrial damage. Additionally, HKL downregulated cGAS expression in senescent ATII cells induced by silica, suggesting sirt3's role as an upstream regulator of the cGAS/STING signaling pathway. Moreover, honokiol treatment inhibited the activation of the NF-κB signaling pathway, associated with reduced oxidative stress and mtDNA damage. Notably, HKL enhanced the activity of SOD2, crucial for mitochondrial function, through sirt3-mediated deacetylation. Additionally, HKL promoted the deacetylation activity of sirt3, further safeguarding mtDNA integrity.
Conclusions: This study uncovers a natural compound, HKL, with significant anti-fibrotic properties through activating sirt3, shedding light on silicosis pathogenesis and treatment avenues.
Keywords: Mitochondrial DNA damage; Senescence; Silicosis; Type II alveolar epithelial cell; sirtuin3.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Honokiol ameliorates silica-induced lung fibrosis by inhibiting macrophage pyroptosis via modulating cGAS/STING signaling.Int Immunopharmacol. 2025 Jan 27;146:113812. doi: 10.1016/j.intimp.2024.113812. Epub 2024 Dec 15. Int Immunopharmacol. 2025. PMID: 39681061
-
Sirtuin-3 activation by honokiol attenuated anesthesia/surgery-induced cognitive impairment and neuronal ferroptosis via inhibiting mitochondrial GPX4 acetylation.J Nanobiotechnology. 2025 Jun 4;23(1):414. doi: 10.1186/s12951-025-03502-y. J Nanobiotechnology. 2025. PMID: 40462120 Free PMC article.
-
Honokiol protects pulmonary microvascular endothelial barrier against lipopolysaccharide-induced ARDS partially via the Sirt3/AMPK signaling axis.Life Sci. 2018 Oct 1;210:86-95. doi: 10.1016/j.lfs.2018.08.064. Epub 2018 Aug 29. Life Sci. 2018. PMID: 30171880
-
Honokiol Targeting SIRT3: From Molecular Mechanisms to Therapeutic Opportunities.FASEB J. 2025 Jul 15;39(13):e70798. doi: 10.1096/fj.202501428R. FASEB J. 2025. PMID: 40590114 Review.
-
The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis.Int J Mol Sci. 2015 Sep 7;16(9):21486-519. doi: 10.3390/ijms160921486. Int J Mol Sci. 2015. PMID: 26370974 Free PMC article. Review.
Cited by
-
A fluorescent STING ligand sensor for high-throughput screening of compounds that can enhance tumor immunotherapy.Cell Rep Methods. 2025 Jul 21;5(7):101106. doi: 10.1016/j.crmeth.2025.101106. Epub 2025 Jul 15. Cell Rep Methods. 2025. PMID: 40669456 Free PMC article.
-
cGAS-STING targeting offers therapy choice in lung diseases.Biol Direct. 2025 Feb 7;20(1):20. doi: 10.1186/s13062-025-00611-4. Biol Direct. 2025. PMID: 39920718 Free PMC article. Review.
-
Pulmonary fibrosis: pathogenesis and therapeutic strategies.MedComm (2020). 2024 Sep 23;5(10):e744. doi: 10.1002/mco2.744. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39314887 Free PMC article. Review.
-
The updated evidence of pirfenidone treated silicosis based on network pharmacology, molecular docking and experimental validation.Front Med (Lausanne). 2025 May 21;12:1573241. doi: 10.3389/fmed.2025.1573241. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40470053 Free PMC article.
-
SIRT3/6/7: promising therapeutic targets for pulmonary fibrosis.Front Cell Dev Biol. 2025 Apr 2;13:1557384. doi: 10.3389/fcell.2025.1557384. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40241794 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

