Minimal and hybrid hydrogenases are active from archaea
- PMID: 38866018
- PMCID: PMC11216029
- DOI: 10.1016/j.cell.2024.05.032
Minimal and hybrid hydrogenases are active from archaea
Abstract
Microbial hydrogen (H2) cycling underpins the diversity and functionality of diverse anoxic ecosystems. Among the three evolutionarily distinct hydrogenase superfamilies responsible, [FeFe] hydrogenases were thought to be restricted to bacteria and eukaryotes. Here, we show that anaerobic archaea encode diverse, active, and ancient lineages of [FeFe] hydrogenases through combining analysis of existing and new genomes with extensive biochemical experiments. [FeFe] hydrogenases are encoded by genomes of nine archaeal phyla and expressed by H2-producing Asgard archaeon cultures. We report an ultraminimal hydrogenase in DPANN archaea that binds the catalytic H-cluster and produces H2. Moreover, we identify and characterize remarkable hybrid complexes formed through the fusion of [FeFe] and [NiFe] hydrogenases in ten other archaeal orders. Phylogenetic analysis and structural modeling suggest a deep evolutionary history of hybrid hydrogenases. These findings reveal new metabolic adaptations of archaea, streamlined H2 catalysts for biotechnological development, and a surprisingly intertwined evolutionary history between the two major H2-metabolizing enzymes.
Keywords: anaerobic; archaea; eukaryogenesis; hydrogen; hydrogenase.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.F.B. is a co-founder of Metagenomi. A patent on this discovery and application of ultraminimal hydrogenases was submitted.
Figures







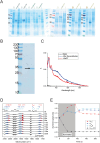


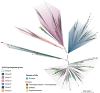

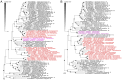
References
-
- Greening C., Biswas A., Carere C.R., Jackson C.J., Taylor M.C., Stott M.B., Cook G.M., Morales S.E. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016;10:761–777. doi: 10.1038/ismej.2015.153. - DOI - PMC - PubMed
-
- Schwartz E., Fritsch J., Friedrich B. In: The Prokaryotes: Prokaryotic Physiology and Biochemistry. Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. Springer; 2013. H2-metabolizing prokaryotes; pp. 119–199. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

