Differential Analysis of Cereblon Neosubstrates in Rabbit Embryos Using Targeted Proteomics
- PMID: 38866076
- PMCID: PMC11263748
- DOI: 10.1016/j.mcpro.2024.100797
Differential Analysis of Cereblon Neosubstrates in Rabbit Embryos Using Targeted Proteomics
Abstract
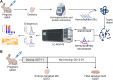
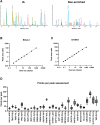
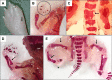
Targeted protein degradation is the selective removal of a protein of interest through hijacking intracellular protein cleanup machinery. This rapidly growing field currently relies heavily on the use of the E3 ligase cereblon (CRBN) to target proteins for degradation, including the immunomodulatory drugs (IMiDs) thalidomide, lenalidomide, and pomalidomide which work through a molecular glue mechanism of action with CRBN. While CRBN recruitment can result in degradation of a specific protein of interest (e.g., efficacy), degradation of other proteins (called CRBN neosubstrates) also occurs. Degradation of one or more of these CRBN neosubstrates is believed to play an important role in thalidomide-related developmental toxicity observed in rabbits and primates. We identified a set of 25 proteins of interest associated with CRBN-related protein homeostasis and/or embryo/fetal development. We developed a targeted assay for these proteins combining peptide immunoaffinity enrichment and high-resolution mass spectrometry and successfully applied this assay to rabbit embryo samples from pregnant rabbits dosed with three IMiDs. We confirmed previously reported in vivo decreases in neosubstrates like SALL4, as well as provided evidence of neosubstrate changes for proteins only examined in vitro previously. While there were many proteins that were similarly decreased by all three IMiDs, no compound had the exact same neosubstrate degradation profile as another. We compared our data to previous literature reports of IMiD-induced degradation and known developmental biology associations. Based on our observations, we recommend monitoring at least a major subset of these neosubstrates in a developmental test system to improve CRBN-binding compound-specific risk assessment. A strength of our assay is that it is configurable, and the target list can be readily adapted to focus on only a subset of proteins of interest or expanded to incorporate new findings as additional information about CRBN biology is discovered.
Keywords: CRBN; IMiD; PRM; developmental biology; developmental toxicity; immunoaffinity; neosubstrate; rabbit embryo.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest All authors are employees of Pfizer, Inc.
Figures


Similar articles
-
Thalidomide Inhibits Human iPSC Mesendoderm Differentiation by Modulating CRBN-dependent Degradation of SALL4.Sci Rep. 2020 Feb 18;10(1):2864. doi: 10.1038/s41598-020-59542-x. Sci Rep. 2020. PMID: 32071327 Free PMC article.
-
Generation of a lenalidomide-sensitive syngeneic murine in vivo multiple myeloma model by expression of CrbnI391V.Exp Hematol. 2021 Jan;93:61-69.e4. doi: 10.1016/j.exphem.2020.11.004. Epub 2020 Nov 11. Exp Hematol. 2021. PMID: 33186626
-
Mapping the IMiD-dependent cereblon interactome using BioID-proximity labelling.FEBS J. 2024 Nov;291(22):4892-4912. doi: 10.1111/febs.17196. Epub 2024 Jul 8. FEBS J. 2024. PMID: 38975872
-
[Development of novel cereblon modulators and their target molecules].Rinsho Ketsueki. 2022;63(6):573-579. doi: 10.11406/rinketsu.63.573. Rinsho Ketsueki. 2022. PMID: 35831190 Review. Japanese.
-
Exploiting ubiquitin ligase cereblon as a target for small-molecule compounds in medicine and chemical biology.Cell Chem Biol. 2021 Jul 15;28(7):987-999. doi: 10.1016/j.chembiol.2021.04.012. Epub 2021 May 24. Cell Chem Biol. 2021. PMID: 34033753 Review.
Cited by
-
Effects of a Diet of Allium Extract on Growth, Biochemistry, Metabolism, and Gut Microbiota of Rabbits (Oryctolagus cuniculus).Foods. 2024 Dec 9;13(23):3976. doi: 10.3390/foods13233976. Foods. 2024. PMID: 39683048 Free PMC article.
References
-
- Fang Y., Wang S., Han S., Zhao Y., Yu C., Liu H., et al. Targeted protein degrader development for cancer: advances, challenges, and opportunities. Trends Pharmacol. Sci. 2023;44:303–317. - PubMed
-
- Matyskiela M.E., Couto S., Zheng X., Lu G., Hui J., Stamp K., et al. SALL4 mediates teratogenicity as a thalidomide-dependent cereblon substrate. Nat. Chem. Biol. 2018;14:981–987. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

