Modeling the structure and DAP5-binding site of the FGF-9 5'-UTR RNA utilized in cap-independent translation
- PMID: 38866431
- PMCID: PMC11331406
- DOI: 10.1261/rna.080013.124
Modeling the structure and DAP5-binding site of the FGF-9 5'-UTR RNA utilized in cap-independent translation
Abstract
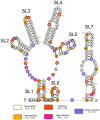
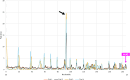
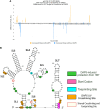
Cap-independent or eukaryotic initiation factor (eIF) 4E-independent, translation initiation in eukaryotes requires scaffolding protein eIF4G or its homolog, death-associated protein 5 (DAP5). eIF4G associates with the 40S ribosomal subunit, recruiting the ribosome to the RNA transcript. A subset of RNA transcripts, such as fibroblast growth factor 9 (FGF-9), contain 5' untranslated regions (5' UTRs) that directly bind DAP5 or eIF4GI. For viral mRNA, eIF recruitment usually utilizes RNA structure, such as a pseudoknot or stem-loops, and the RNA-helicase eIF4A is required for DAP5- or 4G-mediated translation, suggesting these 5' UTRs are structured. However, for cellular IRES-like translation, no consensus RNA structures or sequences have yet been identified for eIF binding. However, the DAP5-binding site within the FGF-9 5' UTR is unknown. Moreover, DAP5 binds to other, dissimilar 5' UTRs, some of which require an unpaired, accessible 5' end to stimulate cap-independent translation. Using SHAPE-seq, we modeled the 186 nt FGF-9 5'-UTR RNA's complex secondary structure in vitro. Further, DAP5 footprinting, toeprinting, and UV cross-linking experiments identify DAP5-RNA interactions. Modeling of FGF-9 5'-UTR tertiary structure aligns DAP5-interacting nucleotides on one face of the predicted structure. We propose that RNA structure involving tertiary folding, rather than a conserved sequence or secondary structure, acts as a DAP5-binding site. DAP5 appears to contact nucleotides near the start codon. Our findings offer a new perspective in the hunt for cap-independent translational enhancers. Structural, rather than sequence-specific, eIF-binding sites may act as attractive chemotherapeutic targets or as dosage tools for mRNA-based therapies.
Keywords: DAP5; DAP5-binding site; FGF-9 RNA; biophysics; cap-independent translation.
© 2024 Whittaker and Goss; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures





Update of
-
Modeling the Structure and DAP5 Binding Site of a Cap-Independent Translational Enhancer mRNA.bioRxiv [Preprint]. 2023 Jun 7:2023.06.07.542187. doi: 10.1101/2023.06.07.542187. bioRxiv. 2023. Update in: RNA. 2024 Aug 16;30(9):1184-1198. doi: 10.1261/rna.080013.124. PMID: 37333283 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
