The Lack of Synapsin Alters Presynaptic Plasticity at Hippocampal Mossy Fibers in Male Mice
- PMID: 38866497
- PMCID: PMC11223178
- DOI: 10.1523/ENEURO.0330-23.2024
The Lack of Synapsin Alters Presynaptic Plasticity at Hippocampal Mossy Fibers in Male Mice
Erratum in
-
Erratum: Bruentgens et al., "The Lack of Synapsin Alters Presynaptic Plasticity at Hippocampal Mossy Fibers in Male Mice".eNeuro. 2024 Sep 13;11(9):ENEURO.0360-24.2024. doi: 10.1523/ENEURO.0360-24.2024. Print 2024 Sep. eNeuro. 2024. PMID: 39271241 Free PMC article. No abstract available.
Abstract
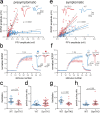
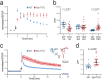
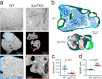
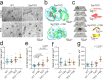
Synapsins are highly abundant presynaptic proteins that play a crucial role in neurotransmission and plasticity via the clustering of synaptic vesicles. The synapsin III isoform is usually downregulated after development, but in hippocampal mossy fiber boutons, it persists in adulthood. Mossy fiber boutons express presynaptic forms of short- and long-term plasticity, which are thought to underlie different forms of learning. Previous research on synapsins at this synapse focused on synapsin isoforms I and II. Thus, a complete picture regarding the role of synapsins in mossy fiber plasticity is still missing. Here, we investigated presynaptic plasticity at hippocampal mossy fiber boutons by combining electrophysiological field recordings and transmission electron microscopy in a mouse model lacking all synapsin isoforms. We found decreased short-term plasticity, i.e., decreased facilitation and post-tetanic potentiation, but increased long-term potentiation in male synapsin triple knock-out (KO) mice. At the ultrastructural level, we observed more dispersed vesicles and a higher density of active zones in mossy fiber boutons from KO animals. Our results indicate that all synapsin isoforms are required for fine regulation of short- and long-term presynaptic plasticity at the mossy fiber synapse.
Keywords: hippocampal mossy fibers; presynaptic plasticity; presynaptic potentiation; synapsin; synaptic transmission; synaptic vesicles.
Copyright © 2024 Bruentgens et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
