Neural Filtering of Physiological Tremor Oscillations to Spinal Motor Neurons Mediates Short-Term Acquisition of a Skill Learning Task
- PMID: 38866498
- PMCID: PMC11255391
- DOI: 10.1523/ENEURO.0043-24.2024
Neural Filtering of Physiological Tremor Oscillations to Spinal Motor Neurons Mediates Short-Term Acquisition of a Skill Learning Task
Abstract
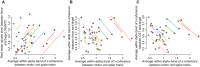
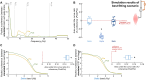
The acquisition of a motor skill involves adaptations of spinal and supraspinal pathways to alpha motoneurons. In this study, we estimated the shared synaptic contributions of these pathways to understand the neural mechanisms underlying the short-term acquisition of a new force-matching task. High-density surface electromyography (HDsEMG) was acquired from the first dorsal interosseous (FDI; 7 males and 6 females) and tibialis anterior (TA; 7 males and 4 females) during 15 trials of an isometric force-matching task. For two selected trials (pre- and post-skill acquisition), we decomposed the HDsEMG into motor unit spike trains, tracked motor units between trials, and calculated the mean discharge rate and the coefficient of variation of interspike interval (COVISI). We also quantified the post/pre ratio of motor units' coherence within delta, alpha, and beta bands. Force-matching improvements were accompanied by increased mean discharge rate and decreased COVISI for both muscles. Moreover, the area under the curve within alpha band decreased by ∼22% (TA) and ∼13% (FDI), with no delta or beta bands changes. These reductions correlated significantly with increased coupling between force/neural drive and target oscillations. These results suggest that short-term force-matching skill acquisition is mediated by attenuation of physiological tremor oscillations in the shared synaptic inputs. Supported by simulations, a plausible mechanism for alpha band reductions may involve spinal interneuron phase-cancelling descending oscillations. Therefore, during skill learning, the central nervous system acts as a matched filter, adjusting synaptic weights of shared inputs to suppress neural components unrelated to the specific task.
Keywords: common synaptic input; force control; motor unit; skill learning.
Copyright © 2024 Cabral et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








Similar articles
-
Muscle contractile properties directly influence shared synaptic inputs to spinal motor neurons.J Physiol. 2024 Jun;602(12):2855-2872. doi: 10.1113/JP286078. Epub 2024 May 6. J Physiol. 2024. PMID: 38709959
-
Motor unit discharge and force tremor in skill- and strength-trained individuals.Exp Brain Res. 1998 Mar;119(1):27-38. doi: 10.1007/s002210050316. Exp Brain Res. 1998. PMID: 9521533 Clinical Trial.
-
Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans.J Physiol. 2006 Jun 15;573(Pt 3):843-55. doi: 10.1113/jphysiol.2006.105361. Epub 2006 Mar 31. J Physiol. 2006. PMID: 16581867 Free PMC article.
-
Corticospinal and spinal adaptations to motor skill and resistance training: Potential mechanisms and implications for motor rehabilitation and athletic development.Eur J Appl Physiol. 2021 Mar;121(3):707-719. doi: 10.1007/s00421-020-04584-2. Epub 2021 Jan 2. Eur J Appl Physiol. 2021. PMID: 33389142 Review.
-
Human spinal cord injury: motor unit properties and behaviour.Acta Physiol (Oxf). 2014 Jan;210(1):5-19. doi: 10.1111/apha.12153. Epub 2013 Sep 13. Acta Physiol (Oxf). 2014. PMID: 23901835 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
