Tractometry of Human Visual White Matter Pathways in Health and Disease
- PMID: 38866532
- PMCID: PMC11234945
- DOI: 10.2463/mrms.rev.2024-0007
Tractometry of Human Visual White Matter Pathways in Health and Disease
Abstract
Diffusion-weighted MRI (dMRI) provides a unique non-invasive view of human brain tissue properties. The present review article focuses on tractometry analysis methods that use dMRI to assess the properties of brain tissue within the long-range connections comprising brain networks. We focus specifically on the major white matter tracts that convey visual information. These connections are particularly important because vision provides rich information from the environment that supports a large range of daily life activities. Many of the diseases of the visual system are associated with advanced aging, and tractometry of the visual system is particularly important in the modern aging society. We provide an overview of the tractometry analysis pipeline, which includes a primer on dMRI data acquisition, voxelwise model fitting, tractography, recognition of white matter tracts, and calculation of tract tissue property profiles. We then review dMRI-based methods for analyzing visual white matter tracts: the optic nerve, optic tract, optic radiation, forceps major, and vertical occipital fasciculus. For each tract, we review background anatomical knowledge together with recent findings in tractometry studies on these tracts and their properties in relation to visual function and disease. Overall, we find that measurements of the brain's visual white matter are sensitive to a range of disorders and correlate with perceptual abilities. We highlight new and promising analysis methods, as well as some of the current barriers to progress toward integration of these methods into clinical practice. These barriers, such as variability in measurements between protocols and instruments, are targets for future development.
Keywords: diffusion magnetic resonance imaging; tractography; tractometry; vision; white matter.
Conflict of interest statement
Authors declare that no conflict of interest exists regarding this article.
Figures
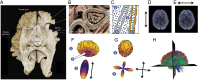
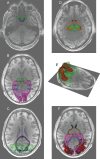
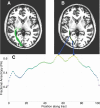
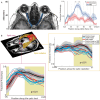
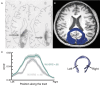
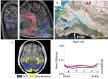
References
-
- Ramrattan RS, Wolfs RC, Panda-Jonas S, et al. Prevalence and causes of visual field loss in the elderly and associations with impairment in daily functioning: The Rotterdam Study. Arch Ophthalmol 2001; 119:1788–1794. - PubMed
-
- Prins D, Hanekamp S, Cornelissen FW. Structural brain MRI studies in eye diseases: Are they clinically relevant? A review of current findings. Acta Ophthalmol 2016; 94:113–121. - PubMed
-
- Azevedo FAC, Carvalho LRB, Grinberg LT, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 2009; 513:532–541. - PubMed
-
- Yakovlev P, Lecours A. The myelogenetic cycles of regional maturation of the brain. In Minkowski A. (ed): Regional development of the brain in early life. Philadelphia, FA Davis Co, 1967, 3-70.

