TWINGEN: protocol for an observational clinical biobank recall and biomarker cohort study to identify Finnish individuals with high risk of Alzheimer's disease
- PMID: 38866570
- PMCID: PMC11177688
- DOI: 10.1136/bmjopen-2023-081947
TWINGEN: protocol for an observational clinical biobank recall and biomarker cohort study to identify Finnish individuals with high risk of Alzheimer's disease
Abstract
Introduction: A better understanding of the earliest stages of Alzheimer's disease (AD) could expedite the development or administration of treatments. Large population biobanks hold the promise to identify individuals at an elevated risk of AD and related dementias based on health registry information. Here, we establish the protocol for an observational clinical recall and biomarker study called TWINGEN with the aim to identify individuals at high risk of AD by assessing cognition, health and AD-related biomarkers. Suitable candidates were identified and invited to participate in the new study among THL Biobank donors according to TWINGEN study criteria.
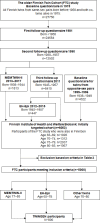
Methods and analysis: A multi-centre study (n=800) to obtain blood-based biomarkers, telephone-administered and web-based memory and cognitive parameters, questionnaire information on lifestyle, health and psychological factors, and accelerometer data for measures of physical activity, sedentary behaviour and sleep. A subcohort is being asked to participate in an in-person neuropsychological assessment (n=200) and wear an Oura ring (n=50). All participants in the TWINGEN study have genome-wide genotyping data and up to 48 years of follow-up data from the population-based older Finnish Twin Cohort (FTC) study of the University of Helsinki. The data collected in TWINGEN will be returned to THL Biobank from where it can later be requested for other biobank studies such as FinnGen that supported TWINGEN.
Ethics and dissemination: This recall study consists of FTC/THL Biobank/FinnGen participants whose data were acquired in accordance with the Finnish Biobank Act. The recruitment protocols followed the biobank protocols approved by Finnish Medicines Agency. The TWINGEN study plan was approved by the Ethics Committee of Hospital District of Helsinki and Uusimaa (number 16831/2022). THL Biobank approved the research plan with the permission no: THLBB2022_83.
Keywords: Dementia; GENETICS; Observational Study.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AP is the Chief Scientific Officer of the FinnGen project that is funded by 13 pharmaceutical companies. HR was a full-time employee of Biogen during study planning and manuscript drafting and has stocks at Merck & Co and Biogen. MMF has received development funding from the Regional Council of Northern Savo and Business Finland for a data-driven tool related to memory disorders and healthcare decision tools, Charles River DRS Finland Ltd. and Orion Pharma have donated equipment for nonclinical cognition testing at the University of Eastern Finland. PI-M has received funding from Orion Research Foundation and Helsinki Biomedicum Foundation outside the present work. RK declares funding paid to the institution from Academy of Finland, 27 Government research funding, Saastamoinen Foundation, Vaajasalo foundation and Jane and Aatos Erkko foundation outside the present work; consulting fees from Orion Pharma; payment or honoraria from Angelini Pharma, Jazz Pharma, Lundbeck, Eisai, Orion Pharma, OmaMedical, Takeda, UCB; participation in monitoring or advisory board from Marinus Pharma and UCB; and leadership or fiduciary role in European Academy of Neurology Epilepsy scientific panel management group, European Epilepsy Reference network Epicare Steering Group and International League Against Epilepsy Career Development Commission. RS declares stocks or stock options at Orion Pharma. S-KH declares payment or honoraria and support for attending meetings or travel from Roche, consulting fees and participation in monitoring or advisory board from Novartis. TK declares payment or honoraria from Novartis Finland and Bayer Nordic SE. The authors declare no other competing financial or non-financial Interests.
Figures
Update of
-
TWINGEN - protocol for an observational clinical biobank recall and biomarker study to identify individuals with high risk of Alzheimer's disease.medRxiv [Preprint]. 2023 Nov 7:2023.11.03.23298018. doi: 10.1101/2023.11.03.23298018. medRxiv. 2023. Update in: BMJ Open. 2024 Jun 12;14(6):e081947. doi: 10.1136/bmjopen-2023-081947. PMID: 37965200 Free PMC article. Updated. Preprint.
References
-
- Official Statistics of Finland (OSF) . Causes of death. Helsinki: Statistics Finland; 2023. Available: https://www.stat.fi/en/statistics/ksyyt [Accessed 29 Sep 2023].
-
- Malzbender K, Lavin-Mena L, Hughes L, et al. . USC Schaeffer Center for Health Policy and Economics; Key Barriers to Clinical Trials for Alzheimer’s Disease, 2020. Available: https://healthpolicy.usc.edu/research/key-barriers-for-clinical-trialsfo...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical