UBE2D3 facilitates NHEJ by orchestrating ATM signalling through multi-level control of RNF168
- PMID: 38866770
- PMCID: PMC11169547
- DOI: 10.1038/s41467-024-49431-6
UBE2D3 facilitates NHEJ by orchestrating ATM signalling through multi-level control of RNF168
Abstract
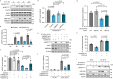
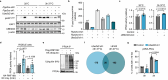
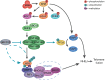
Maintenance of genome integrity requires tight control of DNA damage response (DDR) signalling and repair, with phosphorylation and ubiquitination representing key elements. How these events are coordinated to achieve productive DNA repair remains elusive. Here we identify the ubiquitin-conjugating enzyme UBE2D3 as a regulator of ATM kinase-induced DDR that promotes non-homologous end-joining (NHEJ) at telomeres. UBE2D3 contributes to DDR-induced chromatin ubiquitination and recruitment of the NHEJ-promoting factor 53BP1, both mediated by RNF168 upon ATM activation. Additionally, UBE2D3 promotes NHEJ by limiting RNF168 accumulation and facilitating ATM-mediated phosphorylation of KAP1-S824. Mechanistically, defective KAP1-S824 phosphorylation and telomeric NHEJ upon UBE2D3-deficiency are linked to RNF168 hyperaccumulation and aberrant PP2A phosphatase activity. Together, our results identify UBE2D3 as a multi-level regulator of NHEJ that orchestrates ATM and RNF168 activities. Moreover, they reveal a negative regulatory circuit in the DDR that is constrained by UBE2D3 and consists of RNF168- and phosphatase-mediated restriction of KAP1 phosphorylation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
OTUD5 promotes end-joining of deprotected telomeres by promoting ATM-dependent phosphorylation of KAP1S824.Nat Commun. 2024 Oct 17;15(1):8960. doi: 10.1038/s41467-024-53404-0. Nat Commun. 2024. PMID: 39420004 Free PMC article.
-
ASF1a Promotes Non-homologous End Joining Repair by Facilitating Phosphorylation of MDC1 by ATM at Double-Strand Breaks.Mol Cell. 2017 Oct 5;68(1):61-75.e5. doi: 10.1016/j.molcel.2017.08.021. Epub 2017 Sep 21. Mol Cell. 2017. PMID: 28943310 Free PMC article.
-
USP14 regulates DNA damage repair by targeting RNF168-dependent ubiquitination.Autophagy. 2018;14(11):1976-1990. doi: 10.1080/15548627.2018.1496877. Epub 2018 Aug 10. Autophagy. 2018. PMID: 29995557 Free PMC article.
-
Essential Role of Ubiquitin-Fold Modifier 1 Conjugation in DNA Damage Response.DNA Cell Biol. 2019 Oct;38(10):1030-1039. doi: 10.1089/dna.2019.4861. Epub 2019 Aug 6. DNA Cell Biol. 2019. PMID: 31368785 Review.
-
An insight into understanding the coupling between homologous recombination mediated DNA repair and chromatin remodeling mechanisms in plant genome: an update.Cell Cycle. 2021 Sep;20(18):1760-1784. doi: 10.1080/15384101.2021.1966584. Epub 2021 Aug 26. Cell Cycle. 2021. PMID: 34437813 Free PMC article. Review.
Cited by
-
OTUD5 promotes end-joining of deprotected telomeres by promoting ATM-dependent phosphorylation of KAP1S824.Nat Commun. 2024 Oct 17;15(1):8960. doi: 10.1038/s41467-024-53404-0. Nat Commun. 2024. PMID: 39420004 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- KWF-NKI 2007-3907/KWF Kankerbestrijding (Dutch Cancer Society)
- KWF-NKI 2012-5305/KWF Kankerbestrijding (Dutch Cancer Society)
- KWF 12826/2019-2/KWF Kankerbestrijding (Dutch Cancer Society)
- institutional grant/KWF Kankerbestrijding (Dutch Cancer Society)
- KWF-YIG 11367/KWF Kankerbestrijding (Dutch Cancer Society)
- H2020-MSCA-ITN-2018: 812829/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- institutional grant/Ministerie van Volksgezondheid, Welzijn en Sport (Dutch Ministry of Health, Welfare and Sport)
- Netherlands X-omics Initiative/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organisation for Scientific Research)
- Netherlands X-omics Initiative/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organisation for Scientific Research)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

