MAL2 reprograms lipid metabolism in intrahepatic cholangiocarcinoma via EGFR/SREBP-1 pathway based on single-cell RNA sequencing
- PMID: 38866777
- PMCID: PMC11169275
- DOI: 10.1038/s41419-024-06775-7
MAL2 reprograms lipid metabolism in intrahepatic cholangiocarcinoma via EGFR/SREBP-1 pathway based on single-cell RNA sequencing
Abstract
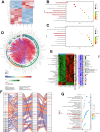
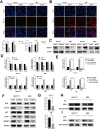
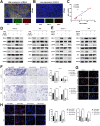
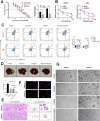
Intrahepatic cholangiocarcinoma (ICC) is a highly aggressive cancer characterized by a poor prognosis and resistance to chemotherapy. In this study, utilizing scRNA-seq, we discovered that the tetra-transmembrane protein mal, T cell differentiation protein 2 (MAL2), exhibited specific enrichment in ICC cancer cells and was strongly associated with a poor prognosis. The inhibition of MAL2 effectively suppressed cell proliferation, invasion, and migration. Transcriptomics and metabolomics analyses suggested that MAL2 promoted lipid accumulation in ICC by stabilizing EGFR membrane localization and activated the PI3K/AKT/SREBP-1 axis. Molecular docking and Co-IP proved that MAL2 interacted directly with EGFR. Based on constructed ICC organoids, the downregulation of MAL2 enhanced apoptosis and sensitized ICC cells to cisplatin. Lastly, we conducted a virtual screen to identify sarizotan, a small molecule inhibitor of MAL2, and successfully validated its ability to inhibit MAL2 function. Our findings highlight the tumorigenic role of MAL2 and its involvement in cisplatin sensitivity, suggesting the potential for novel combination therapeutic strategies in ICC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- 82070676/National Natural Science Foundation of China (National Science Foundation of China)
- 82373265/National Natural Science Foundation of China (National Science Foundation of China)
- 31930020/National Natural Science Foundation of China (National Science Foundation of China)
- 82103430/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

