Using a smartwatch and smartphone to assess early Parkinson's disease in the WATCH-PD study over 12 months
- PMID: 38866793
- PMCID: PMC11169239
- DOI: 10.1038/s41531-024-00721-2
Using a smartwatch and smartphone to assess early Parkinson's disease in the WATCH-PD study over 12 months
Abstract
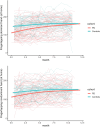
Digital measures may provide objective, sensitive, real-world measures of disease progression in Parkinson's disease (PD). However, multicenter longitudinal assessments of such measures are few. We recently demonstrated that baseline assessments of gait, tremor, finger tapping, and speech from a commercially available smartwatch, smartphone, and research-grade wearable sensors differed significantly between 82 individuals with early, untreated PD and 50 age-matched controls. Here, we evaluated the longitudinal change in these assessments over 12 months in a multicenter observational study using a generalized additive model, which permitted flexible modeling of at-home data. All measurements were included until participants started medications for PD. Over one year, individuals with early PD experienced significant declines in several measures of gait, an increase in the proportion of day with tremor, modest changes in speech, and few changes in psychomotor function. As measured by the smartwatch, the average (SD) arm swing in-clinic decreased from 25.9 (15.3) degrees at baseline to 19.9 degrees (13.7) at month 12 (P = 0.004). The proportion of awake time an individual with early PD had tremor increased from 19.3% (18.0%) to 25.6% (21.4%; P < 0.001). Activity, as measured by the number of steps taken per day, decreased from 3052 (1306) steps per day to 2331 (2010; P = 0.16), but this analysis was restricted to 10 participants due to the exclusion of those that had started PD medications and lost the data. The change of these digital measures over 12 months was generally larger than the corresponding change in individual items on the Movement Disorder Society-Unified Parkinson's Disease Rating Scale but not greater than the change in the overall scale. Successful implementation of digital measures in future clinical trials will require improvements in study conduct, especially data capture. Nonetheless, gait and tremor measures derived from a commercially available smartwatch and smartphone hold promise for assessing the efficacy of therapeutics in early PD.
© 2024. The Author(s).
Conflict of interest statement
Dr. Adams has received research support from the Michael J. Fox Foundation for Parkinson’s Research, Critical Path for Parkinson’s, NIH/NINDS, Biogen, the Huntington Study Group, and PhotoPharmics; received compensation as a consultant/steering committee/advisory board member from the Huntington Study Group, the Parkinson Study Group, AbbVie, VisualDx, BioSensics, Sana Biotechnology, and the Michael J. Fox Foundation for Parkinson’s Research; received honoraria for speaking from the Huntington Study Group, the Parkinson Study Group, American Neurological Association, and Ohio State University. Ms. Tairmae Kangarloo, Dr. Yishu Gong, Dr. Brian Tracey, Dr. Vahe Khachadourian, Dr. Dmitri Volfson, and Dr. Robert Latzman are employees of and own stock in Takeda Pharmaceuticals, Inc. Dr. Josh Cosman is an employee of and owns stock in AbbVie Pharmaceuticals. Dr. Jeremey Edgerton, Dr. Krishna Praneeth Kilambi, and Katherine Fisher are employees of and own stock in Biogen Inc. Dr. Peter R. Bergethon was an employee of Biogen during a portion of this study. He has no conflicts or interests at the present time. Ms. Kostrzebski has received funding from the Michael J. Fox Foundation for Parkinson’s Research, the NIH and the Department of Defense and she holds stock in Apple, Inc. Dr. Dorsey has received compensation for consulting services from Abbott, Abbvie, Acadia, Acorda, Bial-Biotech Investments, Inc., Biogen, Boehringer Ingelheim, California Pacific Medical Center, Caraway Therapeutics, Curasen Therapeutics, Denali Therapeutics, Eli Lilly, Genentech/Roche, Grand Rounds, Huntington Study Group, Informa Pharma Consulting, Karger Publications, LifeSciences Consultants, MCM Education, Mediflix, Medopad, Medrhythms, Merck, Michael J. Fox Foundation, NACCME, Neurocrine, NeuroDerm, NIH, Novartis, Origent Data Sciences, Otsuka, Physician’s Education Resource, Praxis, PRIME Education, Roach, Brown, McCarthy & Gruber, Sanofi, Seminal Healthcare, Spark, Springer Healthcare, Sunovion Pharma, Theravance, Voyager and WebMD; research support from Biosensics, Burroughs Wellcome Fund, CuraSen, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health, Patient-Centered Outcomes Research Institute, Pfizer, PhotoPharmics, Safra Foundation, and Wave LifeSciences; editorial services for Karger Publications; stock in Included Health and in Mediflix, and ownership interests in SemCap. Dr. Espay has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Neuroderm, Neurocrine, Amneal, Acadia, Acorda, Bexion, Kyowa Kirin, Sunovion, Supernus, Avion Pharmaceuticals, and Herantis Pharma; personal compensation as honoraria for speakership for Avion; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer. He cofounded REGAIN Therapeutics (a biotech start-up developing non-aggregating peptide analogs as replacement therapies for neurodegenerative diseases) and is co-owner of a patent that covers synthetic soluble non-aggregating peptide analogs as replacement treatments in proteinopathies. Dr. Spindler has received compensation for consulting services from Medtronic, and clinical trial funding from Abbvie, Abbott, US WorldMeds, Praxis, and Takeda. Dr. Wyant receives research funding from the National Institutes of Health/National Institute of Neurological Disorders and Stroke NeuroNEXT Network, Michael J. Fox Foundation for Parkinson’s Research, Parkinson Study Group, and the Farmer Family Foundation; and royalties from UpToDate. Joan Severson, Allen Best, David Anderson, Michael Merickel, Daniel Jackson Amato, and Brian Severson are employees of Clinical Ink, who acquired the BrainBaseline Platform in 2021 from Digital Artefacts. Joan Severson and Allen Best have financial interests in Clinical Ink. Dr. Chou receives research funding from the Michael J. Fox Foundation, NIH (NS107158), Parkinson Study Group, and Neuraly; received consulting fees from Abbott, Advarra, CVS/Accordant, and Neurocrine, and receives royalties from UpToDate and Springer Publishing. Dr. Shprecher has received research support from Abbvie, Annovis, Biogen, Cognition Therapeutics, Eisai, Jazz Pharmaceuticals Michael J Fox Foundation, Neuraly, Jazz; consulting fees from Amneal, Abbvie, Emalex, and Supernus; and speaker honoraria from Amneal and Neurocrine. Dr. Gunzler has received research funding from the NIH, Michael J. Fox Foundation, Parkinson Study Group, Parkinson Foundation, Biogen, Amneal, and Bial. Dr. Mari has received research funding from the NIH, Michael J. Fox Foundation, Parkinson Study Group, Parkinson Foundation, Cerevel, Amneal, AbbVie, and Neuroderm. Dr. Mari has also received compensation for consulting services from AbbVie, Ipsen, Kyowa Kirin, Amneal, GB Sciences, ACADIA, and Supernus Pharmaceuticals. Dr. Mari is a shareholder of GB Sciences and D&D Pharmatech. Dr. Hogarth received research support from Michael J. Foundation, UCB Biopharma, and the National Institutes of Health. Dr. Barrett receives research funding from the NIH (R21AG077469, R21AG074368) and Kyowa Kirin, Inc. He serves as site PI for clinical trials and studies sponsored by Biogen, CHDI Foundation, Cognition Therapeutics, EIP Pharma, uniQure, Parkinson’s Foundation, and Prilenia Therapeutics.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

