MPXV DNA kinetics in bloodstream and other body fluids samples
- PMID: 38866796
- PMCID: PMC11169222
- DOI: 10.1038/s41598-024-63044-5
MPXV DNA kinetics in bloodstream and other body fluids samples
Abstract
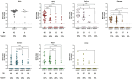
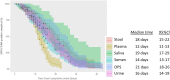
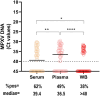
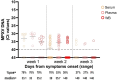
Since spring 2022, the global epidemiology of the monkeypox virus (MPXV) has changed. The unprecedented increase of human clade II MPXV cases worldwide heightened concerns about this emerging zoonotic disease. We analysed the positivity rates, viral loads, infectiousness, and persistence of MPXV DNA for up to 4 months in several biological samples from 89 MPXV-confirmed cases. Our data showed that viral loads and positivity rates were higher during the first two weeks of symptoms for all sample types. Amongst no-skin-samples, respiratory specimens showed higher MPXV DNA levels and median time until viral clearance, suggesting their usefulness in supporting MPXV diagnosis, investigating asymptomatic patients, and monitoring viral shedding. Infectious virus was cultured from respiratory samples, semen, and stools, with high viral loads and collected within the first 10 days. Notably, only one saliva and one semen were found positive for viral DNA after 71 and 31 days from symptoms, respectively. The focus on bloodstream samples showed the best testing sensitivity in plasma, reporting the overall highest MPXV DNA detection rate and viral loads during the 3-week follow-up as compared to serum and whole-blood. The data here presented can be useful for MPXV diagnostics and a better understanding of the potential alternative routes of its onward transmission.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- World Health Organization (WHO). Multi-country outbreak of mpox, External situation report#31–22 December 2023. https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--... (2023).
-
- European Centre for Disease Prevention and Control (ECDC). Outbreak of mpox caused by Monkeypox virus clade I in the Democratic Republic of the Congo. https://www.ecdc.europa.eu/en/news-events/outbreak-mpox-caused-monkeypox... (2024).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

