Understanding the relationship between pore size, surface charge density, and Cu2+ adsorption in mesoporous silica
- PMID: 38866864
- PMCID: PMC11169565
- DOI: 10.1038/s41598-024-64337-5
Understanding the relationship between pore size, surface charge density, and Cu2+ adsorption in mesoporous silica
Abstract
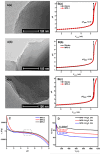
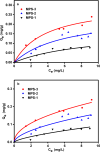
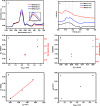
This research delved into the influence of mesoporous silica's surface charge density on the adsorption of Cu2+. The synthesis of mesoporous silica employed the hydrothermal method, with pore size controlled by varying the length of trimethylammonium bromide (CnTAB, n = 12, 14, 16) chains. Gas adsorption techniques and transmission electron microscopy characterized the mesoporous silica structure. Surface charge densities of the mesoporous silica were determined through potentiometric titration, while surface hydroxyl densities were assessed using the thermogravimetric method. Subsequently, batch adsorption experiments were conducted to study the adsorption of Cu2+ in mesoporous silica, and the process was comprehensively analyzed using Atomic absorption spectrometry (AAS), Fourier transform infrared (FTIR), and L3 edge X-ray absorption near edge structure (XANES). The research findings suggest a positive correlation between the pore size of mesoporous silica, its surface charge density, and the adsorption capacity for Cu2+. More specifically, as the pore size increases within the 3-4.1 nm range, the surface charge density and the adsorption capacity for Cu2+ also increase. Our findings provide valuable insights into the relationship between the physicochemical properties of mesoporous silica and the adsorption behavior of Cu2+, offering potential applications in areas such as environmental remediation and catalysis.
Keywords: Cu2+ adsorption; Mesoporous silica; Pore size; Surface charge density.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
In vitro and in vivo evaluation of ordered mesoporous silica as a novel adsorbent in liquisolid formulation.Int J Nanomedicine. 2012;7:199-209. doi: 10.2147/IJN.S26763. Epub 2012 Jan 6. Int J Nanomedicine. 2012. PMID: 22275835 Free PMC article.
-
Synthesis, amino-functionalization of mesoporous silica and its adsorption of Cr(VI).J Colloid Interface Sci. 2008 Feb 15;318(2):309-14. doi: 10.1016/j.jcis.2007.09.093. Epub 2007 Oct 9. J Colloid Interface Sci. 2008. PMID: 18036539
-
Adsorption of copper (II) on mesoporous silica: the effect of nano-scale confinement.Geochem Trans. 2018 Jun 26;19(1):13. doi: 10.1186/s12932-018-0057-4. Geochem Trans. 2018. PMID: 29946861 Free PMC article.
-
Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents.Molecules. 2020 Aug 21;25(17):3814. doi: 10.3390/molecules25173814. Molecules. 2020. PMID: 32825791 Free PMC article. Review.
-
Rice husk waste into various template-engineered mesoporous silica materials for different applications: A comprehensive review on recent developments.Chemosphere. 2023 Jan;310:136843. doi: 10.1016/j.chemosphere.2022.136843. Epub 2022 Oct 12. Chemosphere. 2023. PMID: 36243081 Review.
Cited by
-
Advanced TiO2-Based Photocatalytic Systems for Water Splitting: Comprehensive Review from Fundamentals to Manufacturing.Molecules. 2025 Feb 28;30(5):1127. doi: 10.3390/molecules30051127. Molecules. 2025. PMID: 40076350 Free PMC article. Review.
-
Effects of clean fracturing fluids on coal microstructure and coalbed gas adsorption.Sci Rep. 2024 Sep 3;14(1):20428. doi: 10.1038/s41598-024-71371-w. Sci Rep. 2024. PMID: 39227670 Free PMC article.
References
-
- Hochella MFJE., Jr Nanogeoscience: From origins to cutting-edge applications. Elements. 2008;4(6):373–379. doi: 10.2113/gselements.4.6.373. - DOI
-
- Wang YF. Nanogeochemistry: Nanostructures, emergent properties and their control on geochemical reactions and mass transfers. Chem. Geol. 2014;378:1–23. doi: 10.1016/j.chemgeo.2014.04.007. - DOI
-
- Yuan P, et al. Surface silylation of mesoporous/macroporous diatomite (diatomaceous earth) and its function in Cu(II) adsorption: The effects of heating pretreatment. Microporous Mesoporous Mater. 2013;170:9–19. doi: 10.1016/j.micromeso.2012.11.030. - DOI
-
- Jin JQ, et al. Characterization of natural consolidated halloysite nanotube structures. Minerals. 2021;11:16. doi: 10.3390/min11121308. - DOI
-
- Hochella M, Banfield J. Chemical weathering of silicates in nature: A microscopic perspective with theoretical considerations. In: White AF, Brantley SL, editors. Chemical Weathering Rates of Silicate Minerals. De Gruyter; 2018.
Grants and funding
- 2022BS010/the Doctoral Program Foundation of Guizhou Education University
- QianJiaoHe KY [2022]299/the Youth Science and Technology Talent Development Project of Department of Education of Guizhou Province
- QianJiaoji[2023]021/the Guizhou Provincial University Key Laboratory of Advanced Functional Electronic Materials
LinkOut - more resources
Full Text Sources

