Whole body physiology model to simulate respiratory depression of fentanyl and associated naloxone reversal
- PMID: 38866911
- PMCID: PMC11169242
- DOI: 10.1038/s43856-024-00536-5
Whole body physiology model to simulate respiratory depression of fentanyl and associated naloxone reversal
Abstract
Background: Opioid use in the United States and abroad is an endemic part of society with yearly increases in overdose rates and deaths. In response, the use of the safe and effective reversal agent, naloxone, is being fielded and used by emergency medical technicians at a greater rate. There is evidence that repeated dosing of a naloxone nasal spray is becoming more common. Despite this we lack repeated dosing guidelines as a function of the amount of opiate the patient has taken.
Methods: To measure repeat dosing guidelines, we construct a whole-body model of the pharmacokinetics and dynamics of an opiate, fentanyl on respiratory depression. We then construct a model of nasal deposition and administration of naloxone to investigate repeat dosing requirements for large overdose scenarios. We run a single patient through multiple goal directed resuscitation protocols and measure total naloxone administered.
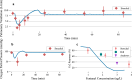
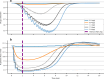
Results: Here we show that naloxone is highly effective at reversing the respiratory symptoms of the patient and recommend dosing requirements as a function of the fentanyl amount administered. We show that for increasing doses of fentanyl, naloxone requirements also increase. The rescue dose displays a nonlinear response to the initial opioid dose. This nonlinear response is largely logistic with three distinct phases: onset, rapid acceleration, and a plateau period for doses above 1.2 mg.
Conclusions: This paper investigates the total naloxone dose needed to properly reverse respiratory depression associated with fentanyl overdose. We show that the current guidelines for a rescue dose may be much lower than required.
Plain language summary
Opioids such as fentanyl are a type of drug that reduce pain. However, the overdose of opioids causes severe breathing issues that can lead to death. Overdose of opioids is an increasing problem across the globe, particularly among people with opioid use disorder. To prevent death, first responders can administer a drug called naloxone that rapidly reverses the effects of opioids. However, the optimum amount of naloxone to administer is unclear. We use a mathematical model to investigate the effect of administering different amounts of naloxone during fentanyl overdose. Our findings suggest that the amount of naloxone to administer that is currently usually administered may be insufficient. Further research should enable naloxone usage guidelines to be optimized, which could improve survival following opioid overdose.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Fact vs. fiction: naloxone in the treatment of opioid-induced respiratory depression in the current era of synthetic opioids.Front Public Health. 2024 Feb 28;12:1346109. doi: 10.3389/fpubh.2024.1346109. eCollection 2024. Front Public Health. 2024. PMID: 38481848 Free PMC article. Review.
-
Pharmacokinetic considerations for community-based dosing of nasal naloxone in opioid overdose in adults.Expert Opin Drug Metab Toxicol. 2022 Mar;18(3):203-217. doi: 10.1080/17425255.2022.2072728. Epub 2022 Jun 8. Expert Opin Drug Metab Toxicol. 2022. PMID: 35500297 Review.
-
Development of a Translational Model to Assess the Impact of Opioid Overdose and Naloxone Dosing on Respiratory Depression and Cardiac Arrest.Clin Pharmacol Ther. 2022 Nov;112(5):1020-1032. doi: 10.1002/cpt.2696. Epub 2022 Jul 22. Clin Pharmacol Ther. 2022. PMID: 35766413
-
Sentanyl: a comparison of blood fentanyl concentrations and naloxone dosing after non-fatal overdose.Clin Toxicol (Phila). 2022 Feb;60(2):197-204. doi: 10.1080/15563650.2021.1948558. Epub 2021 Jul 19. Clin Toxicol (Phila). 2022. PMID: 34278904
-
Real-world study of multiple naloxone administration for opioid overdose reversal among bystanders.Harm Reduct J. 2022 May 20;19(1):49. doi: 10.1186/s12954-022-00627-3. Harm Reduct J. 2022. PMID: 35596213 Free PMC article.
References
LinkOut - more resources
Full Text Sources

