An unbiased ranking of murine dietary models based on their proximity to human metabolic dysfunction-associated steatotic liver disease (MASLD)
- PMID: 38867022
- PMCID: PMC11199145
- DOI: 10.1038/s42255-024-01043-6
An unbiased ranking of murine dietary models based on their proximity to human metabolic dysfunction-associated steatotic liver disease (MASLD)
Abstract
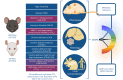
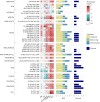
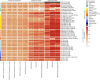
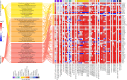
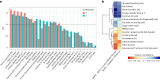
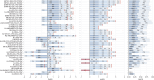
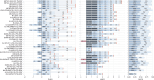
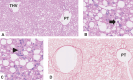
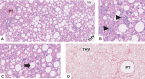
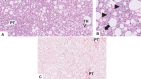
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease, encompasses steatosis and metabolic dysfunction-associated steatohepatitis (MASH), leading to cirrhosis and hepatocellular carcinoma. Preclinical MASLD research is mainly performed in rodents; however, the model that best recapitulates human disease is yet to be defined. We conducted a wide-ranging retrospective review (metabolic phenotype, liver histopathology, transcriptome benchmarked against humans) of murine models (mostly male) and ranked them using an unbiased MASLD 'human proximity score' to define their metabolic relevance and ability to induce MASH-fibrosis. Here, we show that Western diets align closely with human MASH; high cholesterol content, extended study duration and/or genetic manipulation of disease-promoting pathways are required to intensify liver damage and accelerate significant (F2+) fibrosis development. Choline-deficient models rapidly induce MASH-fibrosis while showing relatively poor translatability. Our ranking of commonly used MASLD models, based on their proximity to human MASLD, helps with the selection of appropriate in vivo models to accelerate preclinical research.
© 2024. The Author(s).
Conflict of interest statement
M.V. consults for and receives research funding from Boehringer Ingelheim. J.W.P. is an Eli Lilly and Company employee and may own company stock or possess stock options. A.O. is an employee of Boehringer Ingelheim Pharma. K.G., C.Y., B.B., F.S. and T.R. are Pfizer employees and may own company stock or possess stock options. S.P., M.W.A. and M.F. are employees and own company stocks at Gubra. D.L., A.L., S.O. and A.C.A. are employees of AstraZeneca and may own company stock or possess stock options. F.O. is a director, shareholder and employee of Fibrofind Limited and a director and shareholder in Fibrofind IP Limited. All other authors report they have no conflicts of interest.
Figures








References
-
- Poirier P, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. - DOI - PubMed
MeSH terms
Grants and funding
- CN00000041/Ministero dellapos;Istruzione, dellapos;Universit e della Ricerca (Ministry of Education, University and Research)
- NCI 5P30CA196521-08/U.S. Department of Health Human Services | NIH | NIH Clinical Center (Clinical Center)
- IG27521/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- PTDC/MED-FAR/3492/2021/Fundacin Espaola para la Ciencia y la Tecnologa (Spanish Foundation for Science and Technology)
- PE00000003/Ministero dellapos;Istruzione, dellapos;Universit e della Ricerca (Ministry of Education, University and Research)
- NIH R01 DK136016/U.S. Department of Health Human Services | NIH | NIH Clinical Center (Clinical Center)
- CN00000013/Ministero dellapos;Istruzione, dellapos;Universit e della Ricerca (Ministry of Education, University and Research)
- P202222FCC/Ministero dellapos;Istruzione, dellapos;Universit e della Ricerca (Ministry of Education, University and Research)
- 1948243/RCUK | Medical Research Council (MRC)
- 777377/Innovative Medicines Initiative (IMI)
- MR/K001949/1/MRC_/Medical Research Council/United Kingdom
- R01 DK136016/DK/NIDDK NIH HHS/United States
- R01 DK128289/DK/NIDDK NIH HHS/United States
- MR/R023026/1/RCUK | Medical Research Council (MRC)
- S06-miRNASH/Universita degli Studi di Bari Aldo Moro (University of Bari Aldo Moro)
- MR/K0019494/1/RCUK | Medical Research Council (MRC)
- P30 CA196521/CA/NCI NIH HHS/United States
- NIH R01 DK128289/U.S. Department of Health Human Services | NIH | NIH Clinical Center (Clinical Center)
- Intramural/Foundation for Liver Research
- LCF/PR/HR21/52410028/la Caixa Foundation (Caixa Foundation)

