Strand-resolved mutagenicity of DNA damage and repair
- PMID: 38867042
- PMCID: PMC11186772
- DOI: 10.1038/s41586-024-07490-1
Strand-resolved mutagenicity of DNA damage and repair
Abstract
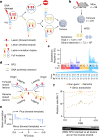
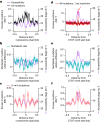
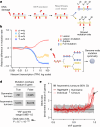
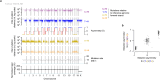
DNA base damage is a major source of oncogenic mutations1. Such damage can produce strand-phased mutation patterns and multiallelic variation through the process of lesion segregation2. Here we exploited these properties to reveal how strand-asymmetric processes, such as replication and transcription, shape DNA damage and repair. Despite distinct mechanisms of leading and lagging strand replication3,4, we observe identical fidelity and damage tolerance for both strands. For small alkylation adducts of DNA, our results support a model in which the same translesion polymerase is recruited on-the-fly to both replication strands, starkly contrasting the strand asymmetric tolerance of bulky UV-induced adducts5. The accumulation of multiple distinct mutations at the site of persistent lesions provides the means to quantify the relative efficiency of repair processes genome wide and at single-base resolution. At multiple scales, we show DNA damage-induced mutations are largely shaped by the influence of DNA accessibility on repair efficiency, rather than gradients of DNA damage. Finally, we reveal specific genomic conditions that can actively drive oncogenic mutagenesis by corrupting the fidelity of nucleotide excision repair. These results provide insight into how strand-asymmetric mechanisms underlie the formation, tolerance and repair of DNA damage, thereby shaping cancer genome evolution.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Error-prone bypass of DNA lesions during lagging-strand replication is a common source of germline and cancer mutations.Nat Genet. 2019 Jan;51(1):36-41. doi: 10.1038/s41588-018-0285-7. Epub 2018 Dec 3. Nat Genet. 2019. PMID: 30510240 Free PMC article.
-
Effects of replication domains on genome-wide UV-induced DNA damage and repair.PLoS Genet. 2022 Sep 26;18(9):e1010426. doi: 10.1371/journal.pgen.1010426. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36155646 Free PMC article.
-
Strand-specific PCR-competitive replication and adduct bypass assay for assessing how DNA adducts perturb DNA replication in mammalian cells.Methods Enzymol. 2024;705:251-270. doi: 10.1016/bs.mie.2024.07.013. Epub 2024 Aug 10. Methods Enzymol. 2024. PMID: 39389666
-
DNA damage response and transcription.DNA Repair (Amst). 2011 Jul 15;10(7):743-50. doi: 10.1016/j.dnarep.2011.04.024. Epub 2011 May 31. DNA Repair (Amst). 2011. PMID: 21622031 Review.
-
Mass Spectrometry-Based Quantitative Strategies for Assessing the Biological Consequences and Repair of DNA Adducts.Acc Chem Res. 2016 Feb 16;49(2):205-13. doi: 10.1021/acs.accounts.5b00437. Epub 2016 Jan 13. Acc Chem Res. 2016. PMID: 26758048 Free PMC article. Review.
Cited by
-
DNA replication timing reveals genome-wide features of transcription and fragility.Nat Commun. 2025 May 19;16(1):4658. doi: 10.1038/s41467-025-59991-w. Nat Commun. 2025. PMID: 40389432 Free PMC article.
-
Evaluation of two algorithms measuring homologous recombination deficiency status in prognostic assessment for treatment-naïve non-small cell lung cancer.Chin J Cancer Res. 2025 Jun 30;37(3):352-364. doi: 10.21147/j.issn.1000-9604.2025.03.05. Chin J Cancer Res. 2025. PMID: 40642492 Free PMC article.
-
Deaminase-Driven Reverse Transcription Mutagenesis in Oncogenesis: Critical Analysis of Transcriptional Strand Asymmetries of Single Base Substitution Signatures.Int J Mol Sci. 2025 Jan 24;26(3):989. doi: 10.3390/ijms26030989. Int J Mol Sci. 2025. PMID: 39940758 Free PMC article. Review.
-
Dynamic crosstalk between amino acid metabolism and cancer drug efficacy: From mechanisms to therapeutic opportunities.iScience. 2025 Apr 11;28(5):112405. doi: 10.1016/j.isci.2025.112405. eCollection 2025 May 16. iScience. 2025. PMID: 40625405 Free PMC article. Review.
-
Disentangling sources of clock-like mutations in germline and soma.bioRxiv [Preprint]. 2023 Sep 12:2023.09.07.556720. doi: 10.1101/2023.09.07.556720. bioRxiv. 2023. Update in: PLoS Biol. 2024 Jun 17;22(6):e3002678. doi: 10.1371/journal.pbio.3002678. PMID: 37745549 Free PMC article. Updated. Preprint.

