Neuroprotective effects of naltrexone in a mouse model of post-traumatic seizures
- PMID: 38867062
- PMCID: PMC11169394
- DOI: 10.1038/s41598-024-63942-8
Neuroprotective effects of naltrexone in a mouse model of post-traumatic seizures
Abstract
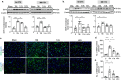
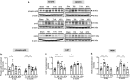
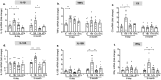
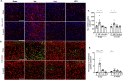
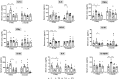
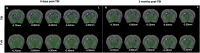
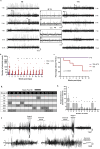
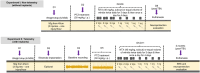
Traumatic Brain Injury (TBI) induces neuroinflammatory response that can initiate epileptogenesis, which develops into epilepsy. Recently, we identified anti-convulsive effects of naltrexone, a mu-opioid receptor (MOR) antagonist, used to treat drug addiction. While blocking opioid receptors can reduce inflammation, it is unclear if post-TBI seizures can be prevented by blocking MORs. Here, we tested if naltrexone prevents neuroinflammation and/or seizures post-TBI. TBI was induced by a modified Marmarou Weight-Drop (WD) method on 4-week-old C57BL/6J male mice. Mice were placed in two groups: non-telemetry assessing the acute effects or in telemetry monitoring for interictal events and spontaneous seizures both following TBI and naltrexone. Molecular, histological and neuroimaging techniques were used to evaluate neuroinflammation, neurodegeneration and fiber track integrity at 8 days and 3 months post-TBI. Peripheral immune responses were assessed through serum chemokine/cytokine measurements. Our results show an increase in MOR expression, nitro-oxidative stress, mRNA expression of inflammatory cytokines, microgliosis, neurodegeneration, and white matter damage in the neocortex of TBI mice. Video-EEG revealed increased interictal events in TBI mice, with 71% mice developing post-traumatic seizures (PTS). Naltrexone treatment ameliorated neuroinflammation, neurodegeneration, reduced interictal events and prevented seizures in all TBI mice, which makes naltrexone a promising candidate against PTS, TBI-associated neuroinflammation and epileptogenesis in a WD model of TBI.
Keywords: Mu-opioid receptors; Naltrexone; Neurodegeneration post-traumatic seizures; Neuroinflammation; Nitro-oxidative stress; Traumatic brain injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Naltrexone is neuroprotective against traumatic brain injury in mu opioid receptor knockout mice.CNS Neurosci Ther. 2021 Jul;27(7):831-841. doi: 10.1111/cns.13655. Epub 2021 May 21. CNS Neurosci Ther. 2021. PMID: 34018697 Free PMC article.
-
Morphine attenuates neuroinflammation and blood-brain barrier disruption following traumatic brain injury through the opioidergic system.Brain Res Bull. 2021 Nov;176:103-111. doi: 10.1016/j.brainresbull.2021.08.010. Epub 2021 Aug 28. Brain Res Bull. 2021. PMID: 34464684
-
Interleukin-1 Receptor in Seizure Susceptibility after Traumatic Injury to the Pediatric Brain.J Neurosci. 2017 Aug 16;37(33):7864-7877. doi: 10.1523/JNEUROSCI.0982-17.2017. Epub 2017 Jul 19. J Neurosci. 2017. PMID: 28724747 Free PMC article.
-
A narrative review of the effects of dexamethasone on traumatic brain injury in clinical and animal studies: focusing on inflammation.Inflammopharmacology. 2023 Dec;31(6):2955-2971. doi: 10.1007/s10787-023-01361-3. Epub 2023 Oct 16. Inflammopharmacology. 2023. PMID: 37843641 Review.
-
Inflammation in epileptogenesis after traumatic brain injury.J Neuroinflammation. 2017 Jan 13;14(1):10. doi: 10.1186/s12974-016-0786-1. J Neuroinflammation. 2017. PMID: 28086980 Free PMC article. Review.
Cited by
-
Reversal of Diabetic Dry Eye by Topical Opioid Receptor Blockade Follows Dual Pathways.Invest Ophthalmol Vis Sci. 2025 Mar 3;66(3):24. doi: 10.1167/iovs.66.3.24. Invest Ophthalmol Vis Sci. 2025. PMID: 40062813 Free PMC article.
-
Role of Central Inflammatory and Oxidative Pathways in the Morphine Exacerbation of Cardiovascular Effects of Sepsis in Rats.Pharmaceuticals (Basel). 2025 Jun 12;18(6):882. doi: 10.3390/ph18060882. Pharmaceuticals (Basel). 2025. PMID: 40573276 Free PMC article.
-
Assessment of Cellular End of Life Management in Acute Brain Injury: Clearing Dying Cells After Injury.Epilepsy Curr. 2024 Sep 28;24(5):353-354. doi: 10.1177/15357597241279767. eCollection 2024 Sep-Oct. Epilepsy Curr. 2024. PMID: 39508006 Free PMC article. No abstract available.
References
-
- Ding, K., Gupta, P. K. & Diaz-Arrastia, R. in Translational Research in Traumatic Brain Injury Frontiers in Neuroscience (eds D. Laskowitz & G. Grant) (2016).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

