PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial
- PMID: 38867077
- PMCID: PMC11186768
- DOI: 10.1038/s41591-024-02979-8
PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial
Abstract
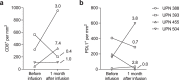
Despite recent therapeutic advances, metastatic castration-resistant prostate cancer (mCRPC) remains lethal. Chimeric antigen receptor (CAR) T cell therapies have demonstrated durable remissions in hematological malignancies. We report results from a phase 1, first-in-human study of prostate stem cell antigen (PSCA)-directed CAR T cells in men with mCRPC. The starting dose level (DL) was 100 million (M) CAR T cells without lymphodepletion (LD), followed by incorporation of LD. The primary end points were safety and dose-limiting toxicities (DLTs). No DLTs were observed at DL1, with a DLT of grade 3 cystitis encountered at DL2, resulting in addition of a new cohort using a reduced LD regimen + 100 M CAR T cells (DL3). No DLTs were observed in DL3. Cytokine release syndrome of grade 1 or 2 occurred in 5 of 14 treated patients. Prostate-specific antigen declines (>30%) occurred in 4 of 14 patients, as well as radiographic improvements. Dynamic changes indicating activation of peripheral blood endogenous and CAR T cell subsets, TCR repertoire diversity and changes in the tumor immune microenvironment were observed in a subset of patients. Limited persistence of CAR T cells was observed beyond 28 days post-infusion. These results support future clinical studies to optimize dosing and combination strategies to improve durable therapeutic outcomes. ClinicalTrials.gov identifier NCT03873805 .
© 2024. The Author(s).
Conflict of interest statement
T.B.D. is a consultant for AstraZeneca and Janssen. S.J.P. and S.J.F. are scientific advisors to and receive royalties from Mustang Bio. S.J.P. is also a scientific advisor and/or receives royalties from Imugene, Adicet Bio, Port Therapeutics and Celularity. S.J.P. and S.J.F. are listed as co-inventors on a patent on chimeric antigen receptors targeted to PSCA, which is owned by the City of Hope. The other authors declare no competing interests.
Figures











References
-
- Kwon ED, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

