Utilising Endogenous Biomarkers in Drug Development to Streamline the Assessment of Drug-Drug Interactions Mediated by Renal Transporters: A Pharmaceutical Industry Perspective
- PMID: 38867094
- PMCID: PMC11222257
- DOI: 10.1007/s40262-024-01385-0
Utilising Endogenous Biomarkers in Drug Development to Streamline the Assessment of Drug-Drug Interactions Mediated by Renal Transporters: A Pharmaceutical Industry Perspective
Abstract
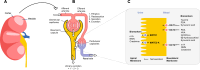
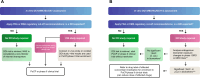
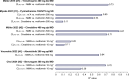
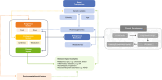
The renal secretion of many drugs is facilitated by membrane transporters, including organic cation transporter 2, multidrug and toxin extrusion protein 1/2-K and organic anion transporters 1 and 3. Inhibition of these transporters can reduce renal excretion of drugs and thereby pose a safety risk. Assessing the risk of inhibition of these membrane transporters by investigational drugs remains a key focus in the evaluation of drug-drug interactions (DDIs). Current methods to predict DDI risk are based on generating in vitro data followed by a clinical assessment using a recommended exogenous probe substrate for the individual drug transporter. More recently, monitoring plasma-based and urine-based endogenous biomarkers to predict transporter-mediated DDIs in early phase I studies represents a promising approach to facilitate, improve and potentially avoid conventional clinical DDI studies. This perspective reviews the evidence for use of these endogenous biomarkers in the assessment of renal transporter-mediated DDI, evaluates how endogenous biomarkers may help to expand the DDI assessment toolkit and offers some potential knowledge gaps. A conceptual framework for assessment that may complement the current paradigm of predicting the potential for renal transporter-mediated DDIs is outlined.
© 2024. The Author(s).
Conflict of interest statement
Hee Jae Choi, Shilpa Madari and Fenglei Huang are full time employees of Boehringer Ingelheim.
Figures
Similar articles
-
Evaluation and Quantitative Prediction of Renal Transporter-Mediated Drug-Drug Interactions.J Clin Pharmacol. 2016 Jul;56 Suppl 7:S110-21. doi: 10.1002/jcph.702. J Clin Pharmacol. 2016. PMID: 27385169 Review.
-
Identification of Endogenous Biomarkers to Predict the Propensity of Drug Candidates to Cause Hepatic or Renal Transporter-Mediated Drug-Drug Interactions.J Pharm Sci. 2017 Sep;106(9):2357-2367. doi: 10.1016/j.xphs.2017.04.007. Epub 2017 Apr 14. J Pharm Sci. 2017. PMID: 28416420 Review.
-
Using Endogenous Biomarkers to Derisk Assessment of Transporter-Mediated Drug-Drug Interactions: A Scientific Perspective.J Clin Pharmacol. 2022 Dec;62(12):1501-1506. doi: 10.1002/jcph.2119. Epub 2022 Jul 19. J Clin Pharmacol. 2022. PMID: 35778968
-
Clinical Probes and Endogenous Biomarkers as Substrates for Transporter Drug-Drug Interaction Evaluation: Perspectives From the International Transporter Consortium.Clin Pharmacol Ther. 2018 Nov;104(5):836-864. doi: 10.1002/cpt.1216. Clin Pharmacol Ther. 2018. PMID: 30347454 Review.
-
Clinical Assessment of Drug Transporter Inhibition Using Biomarkers: Review of the Literature (2015-2024).J Clin Pharmacol. 2025 Jun;65(6):688-703. doi: 10.1002/jcph.6183. Epub 2025 Jan 19. J Clin Pharmacol. 2025. PMID: 39828904 Review.
Cited by
-
Artificial intelligence modeling of biomarker-based physiological age: Impact on phase 1 drug-metabolizing enzyme phenotypes.CPT Pharmacometrics Syst Pharmacol. 2025 Feb;14(2):302-316. doi: 10.1002/psp4.13273. Epub 2024 Nov 14. CPT Pharmacometrics Syst Pharmacol. 2025. PMID: 39540677 Free PMC article.
-
Beyond ADME: The Endogenous Functions of Drug Transporters and Its Impact on Human Disease.Pharmaceutics. 2025 May 23;17(6):685. doi: 10.3390/pharmaceutics17060685. Pharmaceutics. 2025. PMID: 40573999 Free PMC article. Review.
-
Enhancing therapeutic strategies and drug development for patients with kidney disease.Expert Opin Drug Saf. 2025 Jul 4:1-26. doi: 10.1080/14740338.2025.2525970. Online ahead of print. Expert Opin Drug Saf. 2025. PMID: 40568828 Review.
-
Precision medication based on the evaluation of drug metabolizing enzyme and transporter functions.Precis Clin Med. 2025 Feb 22;8(1):pbaf004. doi: 10.1093/pcmedi/pbaf004. eCollection 2025 Mar. Precis Clin Med. 2025. PMID: 40110576 Free PMC article. Review.
-
Is N1-Methylnicotinamide a Good Organic Cation Transporter 2 (OCT2) Biomarker?Metabolites. 2025 Jan 29;15(2):80. doi: 10.3390/metabo15020080. Metabolites. 2025. PMID: 39997705 Free PMC article.
References
-
- US Food and Drug Administration. In vitro drug interaction studies: cytochrome P450 enzyme- and transporter-mediated drug interactions. Guidance for industry. January 2020:1-46. Available from: https://www.fda.gov/media/134582/download. Accessed 25 May 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

